RESEARCH PAPER

Smoking and risk of negative outcomes among COVID-19
patients: A systematic review and meta-analysis
More details
Hide details
1
School of Pharmaceutical Sciences,
University of Phayao, Phayao, Thailand
2
Unit of Excellence on Clinical
Outcomes Research and IntegratioN,
School of Pharmaceutical Sciences,
University of Phayao, Phayao, Thailand
3
Center of Health Outcomes Research
and Therapeutic Safety, School of
Pharmaceutical Sciences, University of
Phayao, Phayao, Thailand
4
Division of Pharmaceutical Care,
Department of Pharmacy, Phrae
Hospital, Phrae, Thailand
5
Department of Global Health and
Development, London School of
Hygiene and Tropical Medicine, London,
United Kingdom
6
Unit of Excellence on Herbal
Medicine, School of Pharmaceutical
Sciences, University of Phayao, Phayao,
Thailand
7
Biofunctional Molecule Exploratory
Research Group, Biomedicine Research
Advancement Centre, School of
Pharmacy, Monash University Malaysia,
Bandar Sunway, Malaysia
8
Novel Bacteria and Drug Discovery
Research Group, Microbiome and
Bioresource Research Strength, Jeffrey
Cheah School of Medicine and Health
Sciences, Monash University Malaysia,
Bandar Sunway, Malaysia
Submission date: 2020-11-09
Final revision date: 2021-01-14
Acceptance date: 2021-01-14
Publication date: 2021-02-04
Corresponding author
Surasak Saokaew
Center of Health
Outcomes Research and Therapeutic
Safety, School of Pharmaceutical
Sciences, University of Phayao, Phayao,
56000 Thailand
Tob. Induc. Dis. 2021;19(February):9
KEYWORDS
TOPICS
ABSTRACT
Introduction:
COVID-19 has major effects on the clinical, humanistic and
economic outcomes among patients, producing severe symptoms and
death. Smoking has been reported as one of the factors that increases
severity and mortality rate among COVID-19 patients. However, the effect
of smoking on such medical outcomes is still controversial. This study
conducted a comprehensive systematic review and meta-analysis (SR/
MA) on the association between smoking and negative outcomes among
COVID-19 patients.
Methods:
Electronic databases, including PubMed, EMBASE, Cochrane
Library, Science Direct, Google Scholar, were systematically searched from
the initiation of the database until 12 December 2020. All relevant studies
about smoking and COVID-19 were screened using a set of inclusion and
exclusion criteria. The Newcastle–Ottawa Scale was used to assess the
methodological quality of eligible articles. Random meta-analyses were
conducted to estimate odds ratios (ORs) with 95% confidence interval
(CIs). Publication bias was assessed using the funnel plot, Begg’s test and
Egger’s test.
Results:
A total of 1248 studies were retrieved and reviewed. A total of
40 studies were finally included for meta-analysis. Both current smoking
and former smoking significantly increase the risk of disease severity
(OR=1.58; 95% CI: 1.16–2.15, p=0.004; and OR=2.48; 95% CI: 1.64–
3.77, p<0.001; respectively) with moderate appearance of heterogeneity.
Similarly, current smoking and former smoking also significantly increase
the risk of death (OR=1.35; 95% CI: 1.12–1.62, p=0.002; and OR=2.58;
95% CI: 2.15–3.09, p<0.001; respectively) with moderate appearance of
heterogeneity. There was no evidence of publication bias, which was tested
by the funnel plot, Begg’s test and Egger’s test.
Conclusions:
Smoking, even current smoking or former smoking, significantly
increases the risk of COVID-19 severity and death. Further causational
studies on this association and ascertianing the underlying mechanisms of
this relation is warranted.
INTRODUCTION
Since December 2019, there has been an outbreak of pneumonia of unknown etiology that was first reported in Wuhan, Hubei Province, China. Following the outbreak, a novel coronavirus SARS-CoV-2 disease, COVID-19, was identified by the World Health Organization (WHO) as the causative virus for the pandemic in China and other parts of the world with more than 30 million cases of infection and 0.9 million deaths globally1. In addition, COVID-19 pandemic caused poor mental health and quality of life, as reported. This pandemic is seen to be far from over and there is a continuing resurgence in many countries. The COVID-19 pandemic has caused panic and anxiety because of the increasing number of COVID-19 cases worldwide2,3. Furthermore, COVID-19 has had a significant global economic impact and a huge burden on healthcare resources4.
Smoking has been assumed to be associated with adverse disease prognosis, as extensive evidence has highlighted the negative impact of tobacco use on lung health. It is also found to be detrimental to the immune system and its responsiveness to infections, making smokers more vulnerable to infectious diseases5. Smoking increases the risk and severity of pulmonary infections because of damage to upper airways and a decrease in pulmonary immune function6. It still remains controversial, however, if smoking results in severe symptoms and death among COVID-19 patients. Some previous studies reported a significant association between current smoking, former versus never smoking with COVID-19 negative outcomes7–10. The differences between risk of severity and death between former and never smoker COVID-19 patients have not been shown11–13. Because of small sample sizes included in these previous studies and differing definitions of disease severity, existing systematic reviews and meta-analyses found limited evidence suggesting that the risk of COVID-19 infection maybe lower among smokers compared to non-smokers, albeit from highly heterogeneous studies14–18.
There were a number of factors related to the severity of COVID-19 and the mortality rate, including: older age (>65 years), comorbidities (e.g. hypertension, diabetes), organ dysfunction, lymphopenia, high cytokines, and weak immune responses19–22. Especially, older age was associated with a dramatically higher risk of severe COVID-19. For example, the case fatality rate in three databases exceeded 1% around the age of 50–55 years, but was 10% above 80–85 years (≥70 years in Italy)23. Males aged >65 years, and smoking patients, face greater risk of developing a severe or critical condition19. A previous meta-analysis showed that all age groups had significantly higher mortality compared to their immediately younger age group, with the largest increase in mortality risk observed in patients with ages 60–69 compared to 50–59 years24. This fact could be influenced by both the aging process and the high prevalence in frailty and comorbidities among the older people, which contribute to a decrease in their functional capacity.
Given the unclear evidence about smoking in COVID-19 infected patients aged ≤65 years, we conducted a comprehensive SR/MA to determine the association between smoking and disease severity in COVID-19 infected patients by including all eligible studies. Systematic searching of databases for available evidence and careful definition of disease severity was performed for a rigorous summary of the conclusions.
METHODS
Protocol and registration
The systematic review and meta-analysis were performed in line with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement25. This research was registered with PROSPERO (Registration Number CRD42020186638). Patients and the public were not involved in this study. The systematic review and meta-analysis are exempt from ethics approval because data were collected and synthesized from previous studies. The patient data are anonymized and available in the public domain. The authors followed applicable EQUATOR Network (https://www.equator-network.org) guidelines during the conduct of research project.
Data sources and search strategy
To find relevant studies, scientific databases including Embase, PubMed, Science Direct, Google Scholar and Cochrane Library databases were systematically searched from their inception to 12 December 2020. Medical Subject Headings (MeSH) were used whenever applicable. Bibliographic lists of related articles were explored. The search strategy was carried out with the following keywords: [tobacco OR smok*] AND [covid OR coronavirus OR sars cov*] with slight adjustments depending on the database. There was no study design and language restriction. Additionally, extra searches were performed in the reference lists of included studies to avoid missing any article (Supplementary file Table S1).
Study selection
All relevant articles that reported clinical characteristics and epidemiological information on smoking among COVID-19 infected patients were included in the analysis. All articles with any design (randomized controlled trials and observational studies) were included. Animal studies, reviews, commentaries, editorials, expert opinions, letters, conference meeting abstracts, case reports, case series, systematic reviews and meta-analyses were excluded. Studies with the same participants that did not include effect estimates or had insufficient data to measure effect estimates were also eliminated. Articles with explicit involvement with the tobacco industry were excluded.
Outcomes measures
The primary outcome was disease severity among COVID-19 patients with a history of smoking. The secondary outcome was death among COVID-19 patients with a history of smoking. The term ‘disease severity’ includes clinical presentations based on physical examinations and laboratory results, and other medical records, as diagnosed and described by physicians.
Disease severity was defined by any of the following criteria.
Patients who required ICU care26.
Severe case as defined by the American Thoracic Society guidelines for community-acquired pneumonia22.
Severe stage, if any of the following criteria existed:
a) shortness of breath, respiratory rate ≥30 times/min; b) oxygen saturation <93% in resting state; c) PaO2/FiO2 ≤300 mmHg. CT imaging showed significant lesion progression >50% within 24 to 48 h; d) respiratory failure requiring mechanical ventilation; e) shock; and f) complications with other organ failure requiring ICU care27.
Severe cases were patients needed supplemental oxygen therapy28.
Severe cases or patients with Acute Respiratory Distress Syndrome (ARDS) having PaO2/FiO2 ≤300 mmHg29.
Severe or critical patients as defined by the General Office of National Health Commission of China, version 5 (2020)30.
In cases where smoking status did not specify type of smoking, it was taken to be current smoking.
Data extraction and quality assessment
Two investigators (AU and SK) independently screened each title, abstract and full-text article for potentially eligible studies. Discrepancies were resolved by discussions with a third investigator (SS). All extracted data were independently reviewed by two investigators (AU and SK). The following information was extracted from each study: setting, region, design, sample size, demographic characteristics of participants (age, sex), details of intervention/exposure (smoking status: current or former smoker), and details of outcomes (disease severity: severe or critical vs non-severe; death), and number of COVID-19 patients. The quality of individual studies was appraised independently using the Newcastle–Ottawa Scale (NOS)31. The NOS assigns a maximum of 9 points, with studies having a total score of ≥7 defined as high quality.
Statistical analysis
We computed odds ratio (OR) and 95% confidence interval (CI) for each study using the number of smokers (former or current) and never smoker with pre-specified outcomes (severity and death). The pool effects were combined using random-effect model. Heterogeneity was investigated using Cochran’s Q statistic and I2. Cochran’s Q statistic with an alpha value of 0.10 was chosen to designate heterogeneity amongst trials for each analysis. Heterogeneity level was assigned as: I2 >75%, 25–75%, and <25% to indicate high, moderate, and low level, respectively31. In the case where heterogeneity existed, an attempt to explore possible sources of heterogeneity was made. Publication bias was assessed using Begg’s test, Egger’s test, and funnel plot32–34. A p<0.05 in publication bias tests was suggestive of publication bias. When publication bias was found, the trim-and-fill method was used35.
Sensitivity and subgroup analysis
To appraise the robustness of our analysis, the sensitivity analysis for unmeasured confounding was used. Subgroup analyses were conducted by age differences between groups, current and former exposure to smoking, and quality of the studies. Meta-regression analysis was performed using random-effects meta-regression, metareg command in STATA software36, adjusting for study characteristics (covariates) on pooled outcome. The following potential moderator variables: age (>65 years), hypertension and diabetes mellitus were included for meta-regression analysis.
RESULTS
Search results and characteristics of studies included
In the initial search, 1248 articles were retrieved from all databases. Of these, 159 were eliminated that were found to be duplicates. All articles were screened using the title and abstract. After evaluating the abstracts, 937 studies were excluded due to their data being irrelevant to our objective. After evaluating the full text, a total of 40 studies with 369287 COVID-19 infected patients were included in the meta-analysis (Figure 1). The important characteristics and outcomes of the included articles were collated (Table 1). Of 40 articles, 19 were conducted in China21,22,27,29,30,37–50, one in Kuwait26, one in Korea28, one in Mexico51, one in Japan52, two in Spain53,54, three in Italy55–57, and twelve in the USA33,58–68. Most articles were retrospective studies. The mean age of the patients in the included studies was 54.10 years. Nineteen studies defined outcomes as disease severity22,27–30,37–39,42,43,46–50,58,60,62,63. Seventeen studies defined outcomes as death21,33,40,44,45,51–57,59,65–68. Four studies used both disease severity and death26,41,61,64. All studies defined smoking status as current smoker. Eleven studies included former smokers and current smokers22,33,43,44,47,57,58,61–63,67.
Quality assessment
Newcastle–Ottawa scale was used to assess the methodological quality of the 40 studies. Results showed 12 studies receiving ≥7 stars26,44,49,55–57,59–62,64,68, and the remaining studies receiving <7 stars (Supplementary Table S2).
Synthesis of results
The results in younger patients (≤65 years) showed that both current smoking and former smoking significantly increase the risk of disease severity (OR=1.58; 95% CI: 1.16–2.15, p=0.004; and OR=2.48; 95% CI: 1.64–3.77, p<0.001; respectively) (Figure 2A). Moreover, both current smoking and former smoking also significantly increase the mortality risk in COVID-19 patients (OR=1.35; 95% CI: 1.12–1.62, p=0.002; and OR=2.58; 95% CI: 2.15–3.09; p<0.001; respectively) with moderate appearance of heterogeneity (Figure 2B).
Sensitivity and subgroup analyses
These analyses were conducted for patients >65 years. Results showed that both current smoking and former smoking significantly increase the risk of death (OR=1.46; 95% CI: 1.18–1.79, p=0.002; and OR=2.54; 95% CI: 2.10–3.08, p<0.001; respectively) (Figure 3B). There were no studies with patients aged >65 years in severity outcome (Figure 3A). The sensitivity analysis for unmeasured confounding for death outcome remained substantial (OR=1.38; 95% CI: 1.12–1.71, p=0.003). The subgroup results were consistent with the main study results mentioned above. Details are shown in Table 2. Subgroup analyses were conducted using average age groups (≤65, >65 years), age differences between groups, current and former exposure to smoking, and the quality of the studies. Both average age groups had higher death rate than never smoker. For the disease severity among current smokers, the OR for the random-effects model in the different age groups was 1.97 (95% CI: 1.21–3.22, p=0.007) and 1.41 (95% CI: 1.01–1.97, p=0.046) in similar age groups. For the disease severity among former smokers, the OR for the random-effects model in the different age groups was 1.77 ( 95% CI: 1.22–2.58, p=0.003) and 3.05 (95% CI: 1.11–8.37, p=0.030) in similar age groups. For death among current smokers, the OR for the random-effects model in the different age groups was 1.53 (95% CI: 1.23–1.90, p<0.001). For the death among former smokers, the OR for the randomeffects model in the different age groups was 2.54 (95% CI: 2.10–3.08, p<0.001). While the death OR from the random-effects model in the stars ≥7 group (NOS quality of study) was 1.86 (95% CI: 1.35–2.55, p<0.001) and 1.52 (95% CI: 1.14–2.02, p=0.004) for stars <7 group. The severity OR from the randomeffects model in the stars <7 group was 2.17 (95% CI: 1.57–3.00, p<0.001) (Table 2).
Figure 1
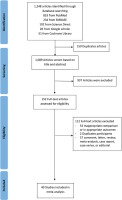
Figure 2
Forest plots showing odds ratio of disease severity (A) and death (B) among younger smokers (≤65 years)
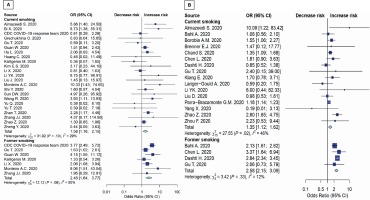
Table 1
General characteristics of 40 studies included
| Author and Year | Location | Study design | Baseline participant characteristics
| Type of smoker a | Outcomes measures | OR (95% CI) | Quality of studies b |
|---|
| Participants | Age (years) Median or Mean (SD) |
|---|
| Almazeedi S. (2020) | Kuwait | Retrospective cohort study | 1096 | 41 | Current | Disease severity Death | 5.86 (1.40–24.47) 10.09 (1.22–83.40) | 7/9 |
| Bahl A. (2020) | USA | Multicenter cohort study | 1461 | 62 | Current Former | Death | 1.08 (0.54–2.04) 2.13 (1.61–2.82) | 6/9 |
| Bellan M. (2020) | Italy | Retrospective study | 312 | 71 | Current | Death | 2.28 (1.18–4.35) | 7/9 |
| Bi X. (2020) | China | Retrospective study | 113 | 46 | Current | Disease severity | 8.73 (1.49–59.80) | 6/9 |
| Borobia A. M. (2020) | Spain | Retrospective study | 2226 | 61 | Current | Death | 1.55 (1.05–2.25) | 6/9 |
| Brenner E.J. (2020) | USA and other countries | Retrospective study | 525 | 41 | Current | Death | 1.47 (0.12–17.53) | 6/9 |
| Castelnuovo A.D. (2020) | Italy | Retrospective observational study | 1842 | 67 (12.96) | Current | Death | 1.09 (0.47–2.49) | 7/9 |
| CDC response team (2020) | USA | Retrospective study | 6637 | ≥19 | Current Former | Disease severity | 0.81 (0.26–1.99) 3.77 (2.46–5.65) | 5/9 |
| Chand S. (2020) | USA | Retrospective study | 300 | 58.2 (12.6) | Current | Death | 1.35 (1.09–1.68) | 6/9 |
| Chen L. (2020) | China | Retrospective study | 1859 | 59 | Current Former | Death | 1.81 (0.87–3.50) 3.37 (1.59–6.74) | 8/9 |
| Dashti H. (2020) | USA | Retrospective study | 12347 | 48 | Current Former | Death | 0.85 (0.51–1.34) 2.84 (2.34–3.46) | 6/9 |
| Grechukhina O. (2020) | USA | Retrospective cohort study | 141 | 30 | Current | Disease severity | 0.83 (0.02–7.11) | 7/9 |
| Gu T. (2020) | USA | Retrospective cohort study | 766 | 47 | Current Current Former Former | Disease severity Death Disease severity Death | 0.59 (0.11–3.23) 2.40 (0.15–39.60) 1.63 (1.02–2.61) 2.06 (0.73–5.77) | 8/9 |
| Guan W. (2020) | China | Retrospective study | 1085 | 47 | Current Former | Disease severity | 1.51 (0.93–2.40) 4.15 (1.51–10.90) | 6/9 |
| Hu L. (2020) | China | Retrospective study | 323 | 61 | Current | Disease severity | 2.06 (0.96–4.66) | 6/9 |
| Huang C. (2020) | China | Retrospective study | 41 | 49 | Current | Disease severity | 0.46 (0.01–5.40) | 6/9 |
| Kalligeros M. (2020) | USA | Retrospective study | 103 | 60 | Current Former | Disease severity | 0.36 (0.06–1.59) 1.33 (0.54–3.24) | 8/9 |
| Kim E.S. (2020) | Korea | Retrospective study | 28 | 42.6 (13.4) | Current | Disease severity | 3.17 (0.19–37.39) | 5/9 |
| Kishaba T. (2020) | Japan | Single-center retrospective cohort study | 7 | 74 | Current | Death | 0.13 (0.00–3.08) | 6/9 |
| Klang E. (2020) | USA | Retrospective study | 572 | 46.5 | Current | Death | 1.70 (0.80–3.80) | 8/9 |
| Langer-Gould A. (2020) | USA | Retrospective cohort study | 93 | 59.3 | Current | Death | 0.59 (0.20–1.68) | 7/9 |
| Li X. (2020) | China | Ambispective cohort study | 548 | 60 | Current Former | Disease severity | 0.81 (0.4–1.61) 2.06 (1.09–3.99) | 6/9 |
| Li YK. (2020) | China | Retrospective study | 25 | 51 | Current | Disease severity Death | 8.75 (0.89–113.30) 6.00 (0.47–87.66) | 6/9 |
| Liu D. (2020) | China | Retrospective study | 599 | 63 | Current | Death | 0.98 (0.52–1.78) | 6/9 |
| Liu J. (2020) | China | Retrospective study | 40 | 48.7 | Current | Disease severity | 1.45 (0.12–14.56) | 6/9 |
| Monteiro A.C. (2020) | USA | Retrospective observational cohort study | 112 | 61 | Current Former | Disease severity | 10.33 (1.43–74.67 8.06 (1.51–43.06) | 6/9 |
| Parra-Bracamonte G. M. (2020) | Mexico | Retrospective study | 331298 | 44 | Current | Death | 1.18 (1.13–1.22) | 6/9 |
| Shi Y. (2020) | China | Retrospective study | 487 | 46 | Current | Disease severity | 1.60 (0.52–4.17) | 6/9 |
| Sun DW. (2020) | China | Retrospective study | 57 | 64 | Current | Disease severity | 4.97 (0.61–227.20) | 6/9 |
| Torres-Macho J. (2020) | Spain | Retrospective observational study | 1968 | 67 | Current | Death | 2.44 (1.89–3.17) | 6/9 |
| Wang R. (2020) | China | Retrospective study | 125 | 42 | Current | Disease severity | 3.93 (1.08–13.56) | 6/9 |
| Yang X. (2020) | China | Retrospective observational study | 52 | 51.9 | Current | Death | 0.19 (0.01–2.66) | 6/9 |
| Yu Q. (2020) | China | Multicenter cohort study | 421 | 48 | Current | Disease severity | 0.38 (0.01–2.58) | 7/9 |
| Yu T. (2020) | China | Cross-sectional multicenter clinical study | 95 | 40 (15.88) | Current | Disease severity | 0.39 (0.01–3.40) | 6/9 |
| Zhan T. (2020) | China | Retrospective study | 405 | 56 | Current | Disease severity | 2.28 (1.17–4.47) | 6/9 |
| Zhang JJ. (2020) | China | Retrospective study | 140 | 57 | Current Former | Disease severity | 4.37 (0.34–232.00) 1.95 (0.31–13.78) | 6/9 |
| Zhao Z. (2020) | USA | Retrospective study | 641 | 60 | Current | Death Disease severity | 2.80 (1.64–4.72) 1.30 (0.85–1.97) | 7/9 |
| Zheng Y. (2020) | China | Retrospective study | 73 | 43 | Current | Disease severity | 0.44 (0.04–2.73) | 6/9 |
| Zhou F. (2020) | China | Retrospective cohort study | 191 | 56 | Current | Death | 2.23 (0.51–9.17) | 6/9 |
| Zinellu A. (2020) | Italy | Retrospective study | 94 | 72 | Current Former | Death | 0.88 (0.29–2.55) 0.99 (0.15–4.80) | 7/9 |
Figure 3
Forest plot showing odds ratio of disease severity (A) and death (B) among all age smokers
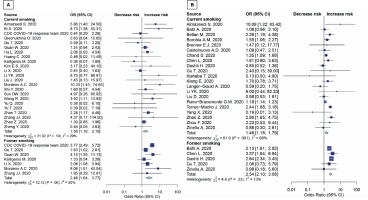
Table 2
Sensitivity and subgroup analyses
| Characteristics | All negative outcomes
| Disease severity
| Death
|
|---|
| OR (95% CI) | Heterogeneity
| OR (95% CI) | Heterogeneity
| OR (95% CI) | Heterogeneity
|
|---|
| I2 (%) | p | I2 (%) | p | I2 (%) | p |
|---|
| Models |
| Fixed effects model | 1.28 (1. 24–1.33) | 75.8 | <0.001 | 1.87 (1.58–2.20) | 44.5 | 0.005 | 1.26 (1.22–1.31) | 83.8 | <0.001 |
| Random effects model | 1.73 (1.45–2.05) | 75.8 | <0.001 | 1.87 (1.43–2.44) | 44.5 | 0.005 | 1.63 (1.30–2.04) | 83.8 | <0.001 |
| Age (years) |
| Overall | 1.73 (1.45–2.05) | 75.8 | <0.001 | 1.87 (1.43–2.44) | 44.5 | 0.005 | 1.63 (1.30–2.04) | 83.8 | <0.001 |
| ≤65 | 1.74 (1.45–2.44) | 75.3 | <0.001 | 1.87 (1.43–2.44) | 44.5 | 0.005 | 1.64 (1.28–2.10) | 84.6 | <0.001 |
| >65 | 1.65 (1.04–2.62) | 45.5 | 0.103 | N/A | N/A | N/A | 1.65 (1.04–2.62) | 45.5 | 0.103 |
| Age different between groups of current smokers |
| Different | 1.61 (1.32–1.96) | 65.0 | <0.001 | 1.97 (1.21–3.22) | 40.3 | 0.072 | 1.53 (1.23–1.90) | 71.0 | <0.001 |
| Similar | 1.25 (0.84–1.88) | 18.6 | 0.266 | 1.41 (1.01–1.97) | 5.4 | 0.390 | 0.52 (0.18–1.48) | 0.0 | <0.001 |
| Age different between groups of former smokers |
| Different | 2.36 (1.97–2.83) | 15.4 | 0.309 | 1.77 (1.22–2.58) | 0.0 | 0.844 | 2.54 (2.10–3.08) | 13.1 | 0.330 |
| Similar | 3.05 (1.11–8.37) | 58.0 | 0.093 | 3.05 (1.11–8.37) | 58.0 | 0.093 | N/A | N/A | N/A |
| Quality of the study (NOS) |
| stars ≥7 | 1.65 (1.28–2.12) | 32.5 | 0.081 | 1.35 (0.93–1.98) | 22.1 | 0.253 | 1.86 (1.35–2.55) | 30.8 | 0.145 |
| stars <7 | 1.79 (1.4–2.23) | 81.7 | <0.001 | 2.17 (1.57–3.00) | 42.6 | 0.019 | 1.52 (1.14–2.02) | 89.7 | <0.001 |
| Omitted unadjusted OR studies |
| Random effects model | 1.38 (1.12–1.71) | 0.8 | 0.402 | N/A | N/A | N/A | 1.38 (1.12–1.71) | 0.8 | 0.402 |
Meta-regression was performed to investigate the following potential moderator variables: age (>65 years), hypertension and diabetes mellitus. No significant moderators of primary and secondary outcomes with studies contributing data emerged, including age >65 years, hypertension, and diabetes mellitus (Supplementary file Table S5).
Publication bias of included studies
An appraisal of publication bias was conducted. There was no apparent publication bias as determined by the symmetric funnel plot, and Begg’s and Egger’s tests revealed no significant difference in all age groups and all outcomes (Supplementary file Figures S1–S6).
DISCUSSION
Summary of evidence
Both current and former smoking significantly increase the risk of disease severity (OR=1.58; 95% CI: 1.16–2.15, p=0.004; and OR=2.48; 95% CI: 1.64–3.77, p<0.001; respectively). Moreover, both current and former smoking also significantly increase the mortality risk among ≤65 years COVID-19 patients (OR=1.35; 95% CI: 1.12–1.62, p=0.002; and OR=2.58; 95% CI: 2.15–3.09, p<0.001; respectively).
We performed a comprehensive SR/MA to assess the possible association between disease severity and death among smokers with COVID-19. According to our analysis, with the biggest sample size, smoking is a risk factor for disease severity and death in COVID-19 patients. Current smokers have 1.58 times the odds of disease severity than never smokers. Remarkably, former smokers have 2.48 times odds of disease severity than never smokers. For death outcome, current and former smoking also significantly increase the risk of death by 1.35 and 2.58 times, respectively.
The most likely mechanism for the potential increase in the risk might be associated with the angiotensin II conversion enzyme-2 (ACE2) receptor, which is in the mucosal epithelial cell and lung alveolar tissue and found to be related to infections with COVID-19. The infection by the host virus attaching to the ACE2 receptors is probably a key step for coronavirus infection. The ACE2 gene expression is heightened in both current and former smokers compared to never smokers in a sample of patients with lung adenocarcinoma, after adjusting for age, gender, and ethnicity5,6,69. This might be a reason why former smokers have higher odds of negative outcomes than never smokers. On the contrary, the findings indicated that current smoking was less likely to have negative outcomes compared with former smoking. These might be due to the following reasons. First, the under-reporting of the current smoking status. Most studies reported smoking history instead of current smoking, which might include former smokers and therefore underestimate current smoking status among COVID-19 patients70. Second, former smokers have longer exposure period or accompanying diseases such as asthma, COPD due to smoking18. As a result, former smoking showed higher risk of negative outcomes compared with current smoking.
Although a previous systematic review examined the association between smoking and overall negative outcomes among COVID-19 patients, it was limited to only Chinese patients12. Another systematic review did not summarize the results as a meta-analysis13. One study demonstrated only the prevalence of smokers among patients hospitalized with COVID-1971 while in another study, the authors retrieved the studies from only one database and the definition of smoking was unclear8. One focused on chronic obstructive pulmonary disease (COPD) and ongoing smoking history17. One meta-analysis included just four selected studies of fair quality, which found that current smokers were more likely to develop severe COVID-19 illness compared to never smokers. But no significant difference was observed between former and never smokers. They also conducted a meta-analysis using two studies deemed to be of fair quality. So they found no significant difference between the risk of death from COVID-19 either between current and never smokers, or former and never smokers11. Finally, all literature collected did not exclude people aged >65 years, which could be a disruptive variable to the study results.
The research question requires well-designed population-based studies that control for age and relevant underlying risk factors. To our best knowledge, this study is the first comprehensive meta-analysis to assess the potential association between former and current smokers and negative outcomes of COVID-19, with the biggest sample size.
Strengths and limitations
This study has several strengths. First, we performed a comprehensive search of major databases (Embase, PubMed, Science Direct, Google Scholar and Cochrane), which is a standard method for conducting a systematic review. Second, we employed a comprehensive search strategy with no restrictions on language and study design. Third, this meta-analysis adheres to the standard methodology of systematic reviews and meta-analyses as required by the PRISMA checklist. Fourth, our study covered updated evidence and was conducted using the appropriate statistical methods for analysis. Finally, the robustness including sensitivity-analysis, subgroup-analysis and meta-regression illustrated that the results remain unchanged.
The study also has some limitations. First, all studies included were observational studies which might have residual confounders; however, this kind of study design reflects a real-world situation for evaluating the association between smoking and disease severity or death in COVID-19 patients. We also used adjusted data from the included studies as much as possible. Nevertheless, there were only non-adjusted data available in some studies. Thus, the residual confounders might distort associations and conclusions. For example, obesity, diabetes, hypertension, asthma and age were reported to increase the risk of severity of COVID-1972–74. We, therefore, analyzed using meta-regression and found that the conclusion remained the same. Second, we searched five major databases, which might not have covered all relevant studies. Nonetheless, after applying Begg’s test, Egger’s test, and a funnel plot, we found no evidence of publication bias. Third, the definitions of severity in each study were slightly different and this is a broad exploratory meta-analysis, which might distort the association between smoking and outcome in COVID-19 patients. Therefore, the results should be interpreted cautiously. However, from another perspective, the effects of smoking in our analysis were consistent across studies, which may indicate high generalizability of the results to any circumstances. Fourth, even key important factors that may potentially affect our findings were number of cigarettes smoked, nicotine addiction level, and the length of time after quitting until COVID-19 infection, which were not reported in the included studies. Nevertheless, our comprehensive sensitivity analysis showed a negative association of smoking on the outcomes.
Further research directions
Well-designed longitudinal population-based studies are needed to address questions about the risk of infection by SARS-CoV-2 and the risk of hospitalization with COVID-19. Stronger evidence coming from smoking status data that are systemically recorded and analyzed among COVID-19 patients are needed. Some factors such as number of cigarettes smoked, nicotine addiction level, and the length of time after quitting until COVID-19 infection should be collected.
CONCLUSIONS
Smoking is confirmed to be a risk factor for the negative progression of COVID-19, particularly on disease severity and death. Both current and former smokers have higher odds of disease severity than never smokers. Given the well-established harm associated with tobacco use, smoking cessation is recommended for all smokers and avoidance of secondhand smoke by non-smokers.
ABBREVIATIONS
COVID-19: coronavirus SARS-CoV-2 disease, OR: odds ratio, CI: confidence interval, WHO: World
Health Organization, GDP: gross domestic product, CVD: cardiovascular disease, MeSH: medical subject headings, ICU:
intensive care unit, PaO2: partial pressure of arterial oxygen, FiO2: inspired oxygen fraction, NOS: Newcastle–Ottawa Scale.
CONFLICTS OF INTEREST
The authors have completed and submitted the ICMJE Form for
Disclosure of Potential Conflicts of Interest and none was reported.
FUNDING
This work was supported by a grant from the Unit of Excellence on
Clinical Outcomes Research and IntegratioN (UNICORN) [Grant number:
FF64-UoE003], School of Pharmaceutical Sciences, University of Phayao.
The funding source had no role in the study design, collection, analysis
and interpretation of data.
AUTHORS' CONTRIBUTIONS
AU, SK and SS contributed to the research idea and design. AU and SS
created the search strategy. AU and SK screened titles, abstracts and full
texts. AU and SK contributed to data extraction and quality assessment.
SK and SS contributed to statistical analysis and interpretation of data.
AU wrote the first draft of the manuscript. SK, SS and DELP edited the
draft of the manuscript. All authors contributed to the critical revision
of the manuscript for important intellectual content, approved and
reviewed the final manuscript.
PROVENANCE AND PEER REVIEW
Not commissioned; externally peer reviewed.
REFERENCES (74)
2.
Qiu J, Shen B, Zhao M, Wang Z, Xie B, Xu Y. A nationwide survey of psychological distress among Chinese people in the COVID-19 epidemic: Implications and policy recommendations. Gen Psychiatr. 2020;33:e100213. doi:10.1136/gpsych-2020-100213.
3.
Zhang Y, Ma ZF. Impact of the COVID-19 pandemic on mental health and quality of life among local residents in Liaoning Province, China: A cross-sectional study. Int J Environ Res Public Health. 2020;17(7):2381. doi:10.3390/ijerph17072381.
5.
Strzelak A, Ratajczak A, Adamiec A, Feleszko W. Tobacco smoke induces and alters immune responses in the lung triggering inflammation, allergy, asthma and other lung diseases: A mechanistic review. Int J Environ Res Public Health. 2018;15(5):1033. doi:10.3390/ijerph15051033.
6.
Arcavi L, Benowitz NL. Cigarette smoking and infection. Arch Intern Med. 2004;164(20):2206-2016. doi:10.1001/archinte.164.20.2206.
7.
Alqahtani JS, Oyelade T, Aldhahir AM, et al. Prevalence, Severity and Mortality associated with COPD and Smoking in patients with COVID-19: A Rapid Systematic Review and Meta-Analysis. PLoS One. 2020;15(5):e0233147. doi:10.1371/journal.pone.0233147.
8.
Patanavanich R, Glantz SA. Smoking is associated with worse outcomes of COVID-19 particularly among younger adults: A systematic review and meta-analysis. Preprint. Posted online on September 23, 2020. medRxiv. doi:10.1101/2020.09.22.20199802.
9.
Kozak R, Prost K, Yip L, Williams V, Leis JA, Mubareka S. Severity of coronavirus respiratory tract infections in adults admitted to acute care in Toronto, Ontario. J Clin Virol. 2020;126:104338. doi:10.1016/j.jcv.2020.104338.
10.
Guo FR. Smoking links to the severity of COVID‐19: An update of a meta‐analysis. J Med Virol. 2020;92(11):2304-2305. doi:10.1002/jmv.25967.
11.
Simons D, Shahab L, Brown J, Perski O. The association of smoking status with SARS-CoV-2 infection, hospitalisation and mortality from COVID-19: A living rapid evidence review (version 4). Preprint. Posted online on June 11, 2020. Qeios. doi:10.32388/UJR2AW.5.
12.
Lippi G, Henry BM. Active smoking is not associated with severity of coronavirus disease 2019 (COVID-19). Eur J Intern Med. 2020;75:107-108. doi:10.1016/j.ejim.2020.03.014.
13.
Vardavas CI, Nikitara K. COVID-19 and smoking: A systematic review of the evidence. Tob Induc Dis. 2020;18(March). doi:10.18332/tid/119324.
14.
Patanavanich R, Glantz SA. The Theoretical Problems Do Not Materially Affect the Results of Our Meta-analysis of Smoking and Covid-19 Disease Progression. Nicotine Tob Res. 2020. doi:10.1093/ntr/ntaa250.
15.
Grundy EJ, Suddek T, Filippidis FT, Majeed A, Coronini-Cronberg S. Smoking, SARS-CoV-2 and COVID-19: A review of reviews considering implications for public health policy and practice. Tob Induc Dis. 2020;18(July). doi:10.18332/tid/124788.
16.
Reddy RK, Charles WN, Sklavounos A, Dutt A, Seed PT, Khajuria A. The effect of smoking on COVID‐19 severity: A systematic review and meta‐analysis. J Med Virol. 2020;93(2). doi:10.1002/jmv.26389.
17.
Zhao Q, Meng M, Kumar R, et al. The impact of COPD and smoking history on the severity of COVID-19: A systemic review and meta-analysis. J Med Virol. 2020;92(10):1915-1921. doi:10.1002/jmv.25889.
18.
Gülsen A, Yigitbas BA, Uslu B, Drömann D, Kilinc O. The Effect of Smoking on COVID-19 Symptom Severity: Systematic Review and Meta-Analysis. Pulm Med. 2020. doi:10.1155/2020/7590207.
19.
Zheng Z, Peng F, Xu B, et al. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. J Infect. 2020;81(2):e16-e25. doi:10.1016/j.jinf.2020.04.021.
20.
Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430-436. doi:10.1038/s41586-020-2521-4.
21.
Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475-481. doi:10.1016/S2213-2600(20)30079-5.
22.
Li X, Xu S, Yu M, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146:110-118. doi:10.1016/j.jaci.2020.04.006.
23.
Onder G, Rezza G, Brusaferro S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA. 2020;1775-1776. doi:10.1001/jama.2020.4683.
24.
Bonanad C, García-Blas S, Tarazona-Santabalbina F, et al. The Effect of Age on Mortality in Patients With COVID-19: A Meta-Analysis With 611,583 Subjects. J Am Med Dir Assoc. 2020;21(7):915-918. doi:10.1016/j.jamda.2020.05.045.
25.
Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339. doi:10.1136/bmj.b2700.
26.
Almazeedi S, Al-Youha S, Jamal MH, et al. Characteristics, risk factors and outcomes among the first consecutive 1096 patients diagnosed with COVID-19 in Kuwait. EClinicalMedicine. 2020;24:100448. doi:10.1016/j.eclinm.2020.100448.
27.
Bi X, Su Z, Yan H, et al. Prediction of severe illness due to COVID-19 based on an analysis of initial Fibrinogen to Albumin Ratio and Platelet count. Platelets. 2020;31:674-679. doi:10.1080/09537104.2020.1760230.
28.
Kim ES, Chin BS, Kang CK, et al. Clinical course and outcomes of patients with severe acute respiratory syndrome coronavirus 2 infection: A preliminary report of the first 28 patients from the Korean Cohort Study on COVID-19. J Korean Med Sci. 2020;35:1-12. doi:10.3346/jkms.2020.35.e142.
29.
Yu T, Cai S, Zheng Z, et al. Association Between Clinical Manifestations and Prognosis in Patients with COVID-19. Clin Ther. 2020;42:964-972. doi: 10.1016/j.clinthera.2020.04.009.
30.
Hu L, Chen S, Fu Y, et al. Risk Factors Associated With Clinical Outcomes in 323 Coronavirus Disease 2019 (COVID-19) Hospitalized Patients in Wuhan, China. Clin Infect Dis. 2020;71:2089-2098. doi:10.1093/cid/ciaa539.
31.
Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. doi:10.1136/bmj.327.7414.557.
32.
Begg CB, Berlin JA. Publication bias and dissemination of clinical research. J Natl Cancer Inst. 1989;81(2):107-115. doi:10.1093/jnci/81.2.107.
33.
Bahl A, Van Baalen MN, Ortiz L, et al. Early predictors of in-hospital mortality in patients with COVID-19 in a large American cohort. Intern Emerg Med. 2020;15(8):1485-1499. doi:10.1007/s11739-020-02509-7.
34.
Sterne JAC, Egger M. Funnel plots for detecting bias in meta-analysis: Guidelines on choice of axis. J Clin Epidemiol. 2001;54(10):1046-1055. doi:10.1016/s0895-4356(01)00377-8.
35.
Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455-463. doi:10.1111/j.0006-341x.2000.00455.x.
36.
Harbord RM, Higgins JPT. Meta-regression in Stata. Stata J. 2008;8:493-519. doi:10.1177/1536867X0800800403.
37.
Sun DW, Zhang D, Tian RH, et al. The underlying changes and predicting role of peripheral blood inflammatory cells in severe COVID-19 patients: A sentinel? Clin Chim Acta. 2020;508:122-129. doi:10.1016/j.cca.2020.05.027.
38.
Shi Y, Yu X, Zhao H, Wang H, Zhao R, Sheng J. Host susceptibility to severe COVID-19 and establishment of a host risk score: Findings of 487 cases outside Wuhan. Crit Care. 2020;24:2-5. doi:10.1186/s13054-020-2833-7.
39.
Liu J, Li S, Liu J, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55. doi:10.1016/j.ebiom.2020.102763.
40.
Liu D, Wang Y, Wang J, et al. Characteristics and outcomes of a sample of patients with COVID-19 identified through social media in Wuhan, China: Observational study. J Med Internet Res. 2020;22(8):e20108. doi:10.2196/20108
41.
Li YK, Peng S, Li LQ, et al. Clinical and Transmission Characteristics of Covid-19 — A Retrospective Study of 25 Cases from a Single Thoracic Surgery Department. Curr Med Sci. 2020;40:295-300. doi:10.1007/s11596-020-2176-2.
42.
Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. doi:10.1016/S0140-6736(20)30183-5.
43.
Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708-1720. doi:10.1056/nejmoa2002032.
44.
Chen L, Yu J, He W, et al. Risk factors for death in 1859 subjects with COVID-19. Leukemia. 2020;34:2173-2183. doi:10.1038/s41375-020-0911-0.
45.
Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054-1062. doi:10.1016/S0140-6736(20)30566-3.
46.
Zheng Y, Xiong C, Liu Y, et al. Epidemiological and clinical characteristics analysis of COVID-19 in the surrounding areas of Wuhan, Hubei Province in 2020. Pharmacol Res. 2020;157:104821. doi:10.1016/j.phrs.2020.104821.
47.
Zhang JJ, Dong X, Cao YY, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75:1730–1741. doi:10.1111/all.14238.
48.
Zhan T, Liu M, Tang Y, et al. Retrospective analysis of clinical characteristics of 405 patients with COVID-19. J Int Med Res. 2020;48(8). doi:10.1177/0300060520949039.
49.
Yu Q, Wang Y, Huang S, et al. Multicenter cohort study demonstrates more consolidation in upper lungs on initial CT increases the risk of adverse clinical outcome in COVID-19 patients. Theranostics. 2020;10:5641-5648. doi:10.7150/thno.46465.
50.
Wang R, Pan M, Zhang X, et al. Epidemiological and clinical features of 125 Hospitalized Patients with COVID-19 in Fuyang, Anhui, China. 2020;95. doi:10.1016/j.ijid.2020.03.070.
51.
Parra-Bracamonte GM, Lopez-Villalobos N, Parra-Bracamonte FE. Clinical characteristics and risk factors for mortality of patients with COVID-19 in a large data set from Mexico. Ann Epidemiol. 2020;52. doi:10.1016/j.annepidem.2020.08.005.
52.
Kishaba T, Maeda A, Nabeya D, Nagano H. Potential Predictors of Poor Prognosis among Critical COVID-19 Pneumonia Patients Requiring Tracheal Intubation. Tohoku J Exp Med. 2020;252(2):103-107. doi:10.1620/tjem.252.103.
53.
Borobia A, Carcas A, Arnalich F, et al. A Cohort of Patients with COVID-19 in a Major Teaching Hospital in Europe. J Clin Med. 2020;9(6):1733. doi:10.3390/jcm9061733.
54.
Torres-Macho J, Ryan P, Valencia J, et al. The PANDEMYC Score. An Easily Applicable and Interpretable Model for Predicting Mortality Associated With COVID-19. J Clin Med. 2020;9. doi:10.3390/jcm9103066.
55.
Bellan M, Patti G, Hayden E, et al. Fatality rate and predictors of mortality in an Italian cohort of hospitalized COVID-19 patients. Sci Rep. 2020;10. doi:10.1038/s41598-020-77698-4.
56.
Di Castelnuovo A, Bonaccio M, Costanzo S, et al. Common cardiovascular risk factors and in-hospital mortality in 3,894 patients with COVID-19: survival analysis and machine learning-based findings from the multicentre Italian CORIST Study. Nutr Metab Cardiovasc Dis. 2020;30:1899-1913. doi:10.1016/j.numecd.2020.07.031.
57.
Zinellu A, Arru F, De Vito A, et al. The De Ritis ratio as prognostic biomarker of in-hospital mortality in COVID-19 patients. Eur J Clin Invest. 2020. doi:10.1111/eci.13427.
58.
CDC COVID-19 Response Team. Preliminary Estimates of the Prevalence of Selected Underlying Health Conditions Among Patients with Coronavirus Disease 2019 — United States, February 1;2–March 28, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(13):382-386. doi:10.15585/mmwr.mm6913e2.
59.
Langer-Gould A, Smith JB, Gonzales EG, et al. Early identification of COVID-19 cytokine storm and treatment with anakinra or tocilizumab. Int J Infect Dis. 2020;99:291-297. doi:10.1016/j.ijid.2020.07.081.
60.
Grechukhina O, Greenberg V, Lundsberg LS, et al. Coronavirus disease 2019 pregnancy outcomes in a racially and ethnically diverse population. Am J Obstet Gynecol MFM. 2020;2(4):100246. doi:10.1016/j.ajogmf.2020.100246.
61.
Gu T, Mack JA, Salvatore M, et al. COVID-19 outcomes, risk factors and associations by race: a comprehensive analysis using electronic health records data in Michigan Medicine. Preprint. Posted online on June 18, 2020. medRxiv. doi:10.1101/2020.06.16.2013314.
62.
Kalligeros M, Shehadeh F, Mylona EK, et al. Association of Obesity with Disease Severity Among Patients with Coronavirus Disease 2019. Obesity. 2020;28(7):1200-1204. doi:10.1002/oby.22859.
63.
Monteiro AC, Suri R, Emeruwa IO, et al. Obesity and Smoking as Risk Factors for Invasive Mechanical Ventilation in COVID-19: a Retrospective, Observational Cohort Study. Preprint. Posted online on August 14, 2020. medRxiv. doi:10.1101/2020.08.12.20173849.
64.
Zhao Z, Chen A, Hou W, et al. Prediction model and risk scores of ICU admission and mortality in COVID-19. PLoS One. 2020;15. doi:10.1371/journal.pone.0236618.
65.
Brenner EJ, Ungaro RC, Gearry RB, et al. Corticosteroids, But Not TNF Antagonists, Are Associated With Adverse COVID-19 Outcomes in Patients With Inflammatory Bowel Diseases: Results From an International Registry. Gastroenterology. 2020;159:481-491.e3. doi:10.1053/j.gastro.2020.05.032.
66.
Chand S, Kapoor S, Orsi D, et al. COVID-19-Associated Critical Illness—Report of the First 300 Patients Admitted to Intensive Care Units at a New York City Medical Center. J Intensive Care Med. 2020;35:963-970. doi:10.1177/0885066620946692.
67.
Dashti H, Roche E, Bates D, Mora S, Demler O. SARS2 simplified scores to estimate risk of hospitalization and death among patients with COVID-19. Preprint. Posted online on September 13, 2020. medRxiv. doi:10.1101/2020.09.11.20190520.
68.
Klang E, Kassim G, Soffer S, Freeman R, Levin MA, Reich DL. Severe Obesity as an Independent Risk Factor for COVID-19 Mortality in Hospitalized Patients Younger than 50. Obesity. 2020;28:1595-1599. doi:10.1002/oby.22913.
69.
Bauer CMT, Morissette MC, Stämpfli MR. The influence of cigarette smoking on viral infections: Translating bench science to impact COPD pathogenesis and acute exacerbations of COPD clinically. Chest. American College of Chest Physicians; 2013;143:196-206. doi:10.1378/chest.12-0930.
70.
Farsalinos K, Barbouni A, Poulas K, Polosa R, Caponnetto P, Niaura R. Current smoking, former smoking, and adverse outcome among hospitalized COVID-19 patients: a systematic review and meta-analysis. Ther Adv Chronic Dis. 2020;11. doi:10.1177/2040622320935765.
71.
Emami A, Javanmardi F, Pirbonyeh N, Akbari A. Prevalence of Underlying Diseases in Hospitalized Patients with COVID-19: a Systematic Review and Meta-Analysis. Arch Acad Emerg Med. 2020;8(1):e35. PMID:32232218.
72.
Singh AK, Gillies CL, Singh R, et al. Prevalence of co-morbidities and their association with mortality in patients with COVID-19: A systematic review and meta-analysis. Diabetes Obes Metab. 2020;22(10):1915-1924. doi:10.1111/dom.14124.
73.
Huang Y, Lu Y, Huang YM, et al. Obesity in patients with COVID-19: a systematic review and meta-analysis. Metabolism. 2020;113. doi:10.1016/j.metabol.2020.154378.
74.
Wang Y, Ao G, Qi X, Xie B. The association between COVID‐19 and asthma: A systematic review and meta‐analysis. Clin Exp Allergy. 2020;50:1274-1277. doi:10.1111/cea.13733.
CITATIONS (83):
1.
Current Activities Centered on Healthy Living and Recommendations for the Future: A Position Statement from the HL-PIVOT Network
Ross Arena, Jonathan Myers, Leonard Kaminsky, Mark Williams, Ahmad Sabbahi, Dejana Popovic, Robert Axtell, Mark Faghy, Andrew Hills, Olivares Olivares, Mildred Lopez, Nicolaas Pronk, Deepika Laddu, Abraham Babu, Richard Josephson, Laurie Whitsel, Rich Severin, Jeffrey Christle, Victor Dourado, Joseph Niebauer, Patrick Savage, Leslie Austford, Carl Lavie
Current Problems in Cardiology
2.
COVID-19 Vaccine Hesitancy and Its Determinants Among Adults with a History of Tobacco or Marijuana Use
Yong Yang, Aram Dobalian, Kenneth Ward
Journal of Community Health
3.
COVID-19 und Rauchen
Matthias Raspe, Robert Bals, Thomas Hering, Wulf Pankow, Alexander Rupp, Christa Rustler, Matthias Urlbauer, Stefan Andreas
Pneumologie
4.
Lower Gene Expression of Angiotensin Converting Enzyme 2 Receptor in Lung Tissues of Smokers with COVID-19 Pneumonia
Francesca Lunardi, Francesco Fortarezza, Luca Vedovelli, Federica Pezzuto, Annalisa Boscolo, Marco Rossato, Roberto Vettor, Anna Cattelan, Vecchio Del, Andrea Crisanti, Paolo Navalesi, Dario Gregori, Fiorella Calabrese
Biomolecules
5.
Smoking and the Risk of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection
Sang Lee, Kang Son, Dong Kim, Chang Han, Yoon Choi, Seong Kim, Seon Park
Nicotine & Tobacco Research
6.
Smokers’ cognitive and behavioural reactions during the early phase of the COVID-19 pandemic: Findings from the 2020 ITC Four Country Smoking and Vaping Survey
Shannon Gravely, Lorraine Craig, K. Cummings, Janine Ouimet, Ruth Loewen, Nadia Martin, Janet Chung-Hall, Pete Driezen, Sara Hitchman, Ann McNeill, Andrew Hyland, Anne Quah, Richard O’Connor, Ron Borland, Mary Thompson, Christian Boudreau, Geoffrey Fong, Stanton Glantz
PLOS ONE
7.
Electronic Cigarette Use Is Not Associated with COVID-19 Diagnosis
Thulasee Jose, Ivana Croghan, J. Hays, Darrell Schroeder, David Warner
Journal of Primary Care & Community Health
8.
Perceived health risks associated with the use of tobacco and
nicotine products during the COVID-19 pandemic
Yong Yang, Eric Lindblom, Ramzi Salloum, Kenneth Ward
Tobacco Induced Diseases
9.
The Risk of COVID-19 Related Hospitalsation, Intensive Care Unit Admission and Mortality in People With Underlying Asthma or COPD: A Systematic Review and Meta-Analysis
Shahina Pardhan, Samantha Wood, Megan Vaughan, Mike Trott
Frontiers in Medicine
10.
What Comes First, the Behavior or the Condition? In the COVID-19 Era, It May Go Both Ways
Ross Arena, Carl Lavie, Mark Faghy
Current Problems in Cardiology
11.
Assessment of Severe COVID-19 Outcomes Using Measures of Smoking Status and Smoking Intensity
E. Mahabee-Gittens, Angelico Mendy, Ashley Merianos
International Journal of Environmental Research and Public Health
12.
The relationship between COVID-19-specific health risk beliefs and the motivation to quit smoking: a UK-based survey
Chris Brown
Drug and Alcohol Dependence
13.
Instigators of COVID-19 in Immune Cells Are Increased in Tobacco Cigarette Smokers and Electronic Cigarette Vapers Compared With Nonsmokers
Theodoros Kelesidis, Yuyan Zhang, Elizabeth Tran, Grace Sosa, Holly Middlekauff
Nicotine & Tobacco Research
14.
Systematic review with meta-analysis of the epidemiological evidence in Europe, Israel, America and Australasia on smoking and COVID-19
Peter Lee, Janette Hamling, Katharine Coombs
World Journal of Meta-Analysis
15.
Risk Factors of In-Hospital Mortality in Non-Specialized Tertiary Center Repurposed for Medical Care to COVID-19 Patients in Russia
Anton Kondakov, Alexander Berdalin, Vladimir Lelyuk, Ilya Gubskiy, Denis Golovin
Diagnostics
16.
Smoking status related to Covid-19 mortality and disease severity in a veteran population
Laura Wilkinson, Kari Mergenhagen, Michael Carter, Hubert Chua, Collin Clark, Bethany Wattengel, John Sellick, Ali El-Solh
Respiratory Medicine
17.
Negative Association Between Smoking and Positive SARS-CoV-2 Testing: Results From a Swiss Outpatient Sample Population
Juan Vallarta-Robledo, José Sandoval, Stéphanie Baggio, Julien Salamun, Frédérique Jacquérioz, Hervé Spechbach, Idris Guessous
Frontiers in Public Health
18.
Impacts of COVID-19 on cigarette use, smoking behaviors, and tobacco purchasing behaviors
Sarah Maloney, Madison Combs, Rebecca Scholtes, Megan Underwood, Barbara Kilgalen, Eric Soule, Alison Breland
Drug and Alcohol Dependence
19.
Mental Well-Being during COVID-19: A Cross-Sectional Study of Fly-In Fly-Out Workers in the Mining Industry in Australia
Bernard Asare, Elizabeth Thomas, Jacquita Affandi, Myles Schammer, Paul Brown, Matthew Pilbeam, Chris Harris, Chris Ellison, Dominika Kwasnicka, Daniel Powell, Christopher Reid, Suzanne Robinson
International Journal of Environmental Research and Public Health
20.
Assessment of Exclusive, Dual, and Polytobacco E-Cigarette Use and COVID-19 Outcomes Among College Students
Ashley Merianos, Alex Russell, E. Mahabee-Gittens, Adam Barry, Meng Yang, Hsien-Chang Lin
American Journal of Health Promotion
21.
Predictors of Mortality Among Hospitalized COVID-19 Patients at a Tertiary Care Hospital in Ethiopia
Galana Ayana, Bedasa Merga, Abdi Birhanu, Addisu Alemu, Belay Negash, Yadeta Dessie
Infection and Drug Resistance
22.
The Exposome and Immune Health in Times of the COVID-19 Pandemic
Javier Morales, Pedro Valenzuela, Adrián Castillo-García, Javier Butragueño, David Jiménez-Pavón, Pedro Carrera-Bastos, Alejandro Lucia
Nutrients
23.
A cross sectional study found differential risks for COVID-19 seropositivity amongst health care professionals in Chile
Marcela Zuñiga, Anne Lagomarcino, Sergio Muñoz, Alfredo Alonso, María Rodriguez, Miguel O'Ryan
Journal of Clinical Epidemiology
24.
The Impact of Tobacco Use on COVID-19 Outcomes: A Systematic Review
Jessica Baker, Nandita Krishnan, Lorien Abroms, Carla Berg, Renee Bittoun
Journal of Smoking Cessation
25.
The Impact of COVID-19 on Brain Stimulation Therapy
Michael Coffey, Suzanne Kerns, Sohag Sanghani, Lee Wachtel
Psychiatric Clinics of North America
26.
Elements and COVID-19: A Comprehensive Overview of Studies on Their Blood/Urinary Levels and Supplementation with an Update on Clinical Trials
Agnieszka Ścibior, Ewa Wnuk
Biology
27.
Israeli News media coverage of COVID-19 and use of cannabis and tobacco: A case study of inconsistent risk communication
Sharon Sznitman, Nehama Lewis
International Journal of Drug Policy
28.
ISPE‐Endorsed Guidance in Using Electronic Health Records for Comparative Effectiveness Research in COVID‐19: Opportunities and Trade‐Offs
Grammati Sarri, Dimitri Bennett, Thomas Debray, Anouk Deruaz‐Luyet, Gabarró Soriano, Joan Largent, Xiaojuan Li, Wei Liu, Jennifer Lund, Daniela Moga, Mugdha Gokhale, Christopher Rentsch, Xuerong Wen, Chen Yanover, Yizhou Ye, Huifeng Yun, Andrew Zullo, Kueiyu Lin
Clinical Pharmacology & Therapeutics
29.
Air Quality and Health [Working Title]
Riccardo Pansini, Lei Shi
30.
Management of patients with SARS-CoV-2 infections with focus on patients with chronic lung diseases (as of 10 January 2022)
Horst Olschewski, Ernst Eber, Brigitte Bucher, Klaus Hackner, Sabin Handzhiev, Konrad Hoetzenecker, Marco Idzko, Walter Klepetko, Gabor Kovacs, Bernd Lamprecht, Judith Löffler-Ragg, Michael Meilinger, Alexander Müller, Christian Prior, Otmar Schindler, Helmut Täubl, Angela Zacharasiewicz, Ralf Zwick, Britt-Madelaine Arns, Josef Bolitschek, Katharina Cima, Elisabeth Gingrich, Maximilian Hochmair, Fritz Horak, Peter Jaksch, Roland Kropfmüller, Andreas Pfleger, Bernhard Puchner, Christoph Puelacher, Patricia Rodriguez, Helmut Salzer, Peter Schenk, Ingrid Stelzmüller, Volker Strenger, Matthias Urban, Marlies Wagner, Franz Wimberger, Holger Flick
Wiener klinische Wochenschrift
31.
The Association of Electronic Cigarette Use With SARS-CoV-2 Infection and COVID-19 Disease Severity
Andrea Burnett-Hartman, Scott Goldberg, J Powers, Morgan Clennin, Jason Lyons, Mark Gray, Heather Feigelson
Tobacco Use Insights
32.
Active Smokers Are at Higher Risk of COVID-19 Death: A Systematic Review and Meta-analysis
Roengrudee Patanavanich, Tanatorn Siripoon, Salin Amponnavarat, Stanton Glantz
Nicotine & Tobacco Research
33.
Changes in cigarette and e-cigarette use among US young
adults from before to during the COVID-19 pandemic: News
exposure and risk perceptions as potential predictors
Breesa Bennett, Katelyn Romm, Carla Berg
Tobacco Prevention & Cessation
34.
Tobacco Smoking and Risk of SARS-CoV-2 Infection and Disease Severity Among Adults in an Integrated Healthcare System in California
Kelly Young-Wolff, Natalie Slama, Stacey Alexeeff, Lori Sakoda, Renee Fogelberg, Laura Myers, Cynthia Campbell, Alyce Adams, Judith Prochaska
Nicotine & Tobacco Research
35.
Smoking patterns during COVID-19: Evidence from Serbia
Jovan Zubović, Aleksandar Zdravković, Olivera Jovanović
Tobacco Induced Diseases
36.
Systematic review of changed smoking behaviour, smoking cessation and psychological states of smokers according to cigarette type during the COVID-19 pandemic
Hae-ryoung Chun, Eunsil Cheon, Ji-eun Hwang
BMJ Open
37.
Smoking increases the risk of post-acute COVID-19
syndrome: Results from a French community-based survey
Hugues Barthélémy, Emmanuelle Mougenot, Martin Duracinsky, Dominique Salmon-Ceron, Jennifer Bonini, Fabienne Péretz, Olivier Chassany, Patrizia Carrieri
Tobacco Induced Diseases
38.
COVID‐19 patients with documented alcohol use disorder or alcohol‐related complications are more likely to be hospitalized and have higher all‐cause mortality
Kristina Bailey, Harlan Sayles, James Campbell, Neha Khalid, Madyson Anglim, Jana Ponce, Todd Wyatt, James McClay, Ellen Burnham, Alfred Anzalone, Corrine Hanson
Alcoholism: Clinical and Experimental Research
39.
Assessment of Risk Factors Associated with COVID-19 Illness Outcomes in a Tertiary Hospital in Saudi Arabia
Mohammad Aljabr, Areej Aldossary, Kanan Alkanani, Zahrani Al, Mulhim Al, Hatim Kheir, Assim AlAbdulkader, Hayat Mushcab, Yaser Alreshidi, Nouf Albalawi, Wedyan Alabdullatif, Abrar Almarzooq, Saeed Qahtani, Jaffar Al-Tawfiq
International Journal of General Medicine
40.
The Impact of the COVID-19 Pandemic on Smoking Consumption: A Systematic Review of Longitudinal Studies
Nerea Almeda, Irene Gómez-Gómez
Frontiers in Psychiatry
41.
Association between smoking and COVID-19 severity: A multicentre retrospective observational study
Yue He, Yangai He, Qinghui Hu, Sheng Yang, Jun Li, Yuan Liu, Jun Hu
Medicine
42.
Changes in Little Cigar and Cigarillo Use during the COVID-19 Pandemic: A Cross-Sectional Analysis of a Nationally Representative Sample of Young Adult Users
Eugenia Lee, Stephanie Moore, Erika Trapl, Craig Fryer, Douglas Gunzler, Kymberle Sterling
International Journal of Environmental Research and Public Health
43.
Predictors of invasive mechanical ventilation in hospitalized COVID-19 patients: a retrospective study from Jordan
Suad Kabbaha, Sayer Al-Azzam, Reema Karasneh, Basheer Khassawneh, Abdel-Hameed Al-Mistarehi, William Lattyak, Motasem Aldiab, Syed Hasan, Barbara Conway, Mamoon Aldeyab
Expert Review of Respiratory Medicine
44.
Associations Between Covid-19-Related Threat, Stress, and Smoking in UK Adults Aged Under- and Over-30
Lucy Walker, Maria Cordero, Gillian McChesney, Ivan Gee, Sarah Grogan
Tobacco Use Insights
45.
Heterogeneous impact of the COVID-19 pandemic on lung, colorectal and breast cancer incidence in Hungary: results from time series and panel data models
Peter Elek, Marcell Csanádi, Petra Fadgyas-Freyler, Nóra Gervai, Rita Oross-Bécsi, Balázs Szécsényi-Nagy, Manna Tatár, Balázs Váradi, Antal Zemplényi
BMJ Open
46.
Impact of a Coronavirus Pandemic on Smoking Behavior in University Students: An Online Survey in Türkiye
Fatma ÇELİK, Göksun DEMİREL
Turkish Journal of Pharmaceutical Sciences
47.
Assessment of COVID-19 Symptoms Distribution According to Tobacco Products Consumption and Khat Chewing: A Potential Antinociceptive Role of Nicotine Among COVID-19 Patients
Ibrahim Gosadi, Ebrahim Abulqusim, Abdulrahman Atiah, Bander Ageeli, Doa'a Alhazmi, Marwah Hamzi, Sara Somaily
International Journal of General Medicine
48.
Changes in Cigarette Smoking and Smokeless Tobacco Use During the Coronavirus Disease 2019 Lockdown Period Among Youth and Young Adults in Denmark
Lotus Bast, Simone Kjeld, Marie Klitgaard
Nicotine & Tobacco Research
49.
The Impact of Smoking Status and Smoking-Related Comorbidities on Coronavirus Disease 2019 Patient Outcomes: A Causal Mediation Analysis
Guen Le, Kelsey Muir, Melanie Simons, Donna Coffman, Rohit Soans
Nicotine & Tobacco Research
50.
Smoking and smoking addiction in future physicians during the COVID-19 pandemic; an example of a medical school in Turkey
Hatice Akbayram, Sibel Dogru
Journal of Substance Use
51.
COVID-19: reducing the risk via diet and lifestyle
Jessica Campbell
Journal of Integrative Medicine
52.
Association of body mass index with COVID-19-related neurologic sequelae: a retrospective cohort study
Sameer Elsayed, Ana Cabrera, Danielle Ouellette, Phil Jones, Rita Dhami, William Hanage
Clinical and Experimental Medicine
53.
Epidemiology, symptomatology, and risk factors for long COVID symptoms: Multi-centre study (Preprint)
Martin Wong, Junjie Huang, Nellie Wong, Grace Wong, Terry Yip, Rachel Chan, Steven Chau, Siew-Chien Ng, Yun-Kwok Wing, Francis Chan
JMIR Public Health and Surveillance
54.
Risk Factors of Severe COVID-19: A Review of Host, Viral and Environmental Factors
Levente Zsichla, Viktor Müller
Viruses
55.
Recognizing risk factors associated with poor outcomes among patients with COVID-19
Paula Rodriguez-Miguelez, Allison Heefner, Salvatore Carbone
Progress in Cardiovascular Diseases
56.
Rationing scarce healthcare capacity: A study of the ventilator allocation guidelines during the COVID‐19 pandemic
David Anderson, Tolga Aydinliyim, Margrét Bjarnadóttir, Eren Çil, Michaela Anderson
Production and Operations Management
57.
Effects of tobacco product use on oral health and the role of
oral healthcare providers in cessation: A narrative review
Sangeeta Gajendra, Scott McIntosh, Sucharu Ghosh
Tobacco Induced Diseases
58.
Ectodomain shedding of proteins important for SARS-CoV-2 pathogenesis in plasma of tobacco cigarette smokers compared to electronic cigarette vapers: a cross-sectional study
Theodoros Kelesidis, Madhav Sharma, Sandro Satta, Elizabeth Tran, Rajat Gupta, Jesus Araujo, Holly Middlekauff
Journal of Molecular Medicine
59.
Modifiable risk factors of COVID-19 in patients with multiple sclerosis: a single-centre case–control study
Federico Montini, Agostino Nozzolillo, Paola Rancoita, Chiara Zanetta, Lucia Moiola, Federica Cugnata, Federica Esposito, Maria Rocca, Vittorio Martinelli, Massimo Filippi
Journal of Neurology
60.
The association between tobacco smoking, second-hand smoke and novel products, and COVID-19 severity and mortality in Italy. Results from the COSMO-IT study
Silvano Gallus, Cristina Bosetti, Giuseppe Gorini, Chiara Stival, Roberto Boffi, Alessandra Lugo, Giulia Carreras, Chiara Veronese, Claudia Santucci, Roberta Pacifici, Biagio Tinghino, Vincenzo Zagà, Patrizia Russo, Maria Cattaruzza
Journal of Epidemiology
61.
Rationing Scarce Healthcare Capacity: A Study Of The Ventilator Allocation Guidelines During The COVID-19 Pandemic In The United States
David Anderson, Tolga Aydinliyim, Margret Bjarnadottir, Eren Cil, Michaela Anderson
SSRN Electronic Journal
62.
Epidemiology, Symptomatology, and Risk Factors for Long COVID Symptoms: Population-Based, Multicenter Study (Preprint)
Martin Wong, Junjie Huang, Yuet-Yan Wong, Grace Wong, Terry Yip, Rachel Chan, Steven Chau, Siew-Chien Ng, Yun-Kwok Wing, Francis Chan
63.
The role of smoking in COVID-19 progression: a comprehensive meta-analysis
Silvano Gallus, Marco Scala, Irene Possenti, Carlotta Jarach, Luke Clancy, Esteve Fernandez, Giuseppe Gorini, Giulia Carreras, Maria Malevolti, Alison Commar, Ranti Fayokun, Hebe Gouda, Vinayak Prasad, Alessandra Lugo
European Respiratory Review
64.
Smoking Suppresses the Therapeutic Potential of Adipose Stem Cells in Crohn’s Disease Patients through Epigenetic Changes
Albert Boronat-Toscano, Irene Vañó, Diandra Monfort-Ferré, Margarita Menacho, Gemma Valldosera, Aleidis Caro, Beatriz Espina, Maria Mañas, Marc Marti, Eloy Espin, Alfonso Saera-Vila, Carolina Serena
Cells
65.
The pathogenetic influence of smoking on SARS-CoV-2 infection: Integrative transcriptome and regulomics analysis of lung epithelial cells
Md. Hossain, Tania Asa, Md. Auwul, Md. Aktaruzzaman, Md. Rahman, Mohammad Moni
Computers in Biology and Medicine
66.
The impact of perceived risk of COVID-19 from smoking on the change in number of cigarettes smoked
Kumar Selva, Haruka Minami
Journal of Substance Use
67.
The association between risk perceptions, anxiety, and self-reported changes in tobacco and nicotine product use due to COVID-19 in May-June 2020 in Israel
Noah Rubinson, Geoffrey Fong, Shannon Gravely, Anne Quah, Michal Bitan, Ari Lev, Laura Rosen
BMC Public Health
68.
Current tobacco smoking and risk of SARS-CoV-2 infection and hospitalization: Evaluating the role of socio-demographic factors and comorbidities
Kelly Young-Wolff, Natalie Slama, Lori Sakoda, Judith Prochaska, Renee Fogelberg, Stacey Alexeeff
Preventive Medicine
69.
Effects of Smoking on COVID-19 Management and Mortality: An Umbrella Review
SeyedAhmad SeyedAlinaghi, Amir Afsahi, Ramin Shahidi, Shaghayegh Kianzad, Zahra Pashaei, Maryam Mirahmad, Pooria Asili, Hengameh Mojdeganlou, Armin Razi, Paniz Mojdeganlou, Iman Fard, Sara Mahdiabadi, Arian Afzalian, Mohsen Dashti, Afsaneh Ghasemzadeh, Zohal Parmoon, Hajar Badri, Esmaeil Mehraeen, Daniel Hackett, Haniki Mohamed
Journal of Smoking Cessation
70.
The controversial effect of smoking and nicotine in SARS-CoV-2 infection
Zahra Salehi, Ghoochani Motlagh, Nourian Hasani, Sadegh Jamalkandi, Mostafa Ghanei
Allergy, Asthma & Clinical Immunology
71.
The predisposition of smokers to COVID-19 infection: A mini-review of global perspectives
Suhana Chattopadhyay, Leena Malayil, Syeda Kaukab, Zachary Merenstein, Amy Sapkota
Heliyon
72.
Changes in smoking due to COVID-19 pandemic among persons of migrant origin compared with the general population: a population-based study
Otto Ruokolainen, Eero Lilja, Hanna Ollila, Anu Castaneda, Päivikki Koponen, Natalia Skogberg
Scandinavian Journal of Public Health
73.
Smoking Predictor for Covid-19 Severity and Mortality- A Systematic Review Based on Evidence for Clinical Practice
Santi Martini, Arief Hargono, Kurnia Artanti, János Sándor, Azizuddin Khan, Besral Besral, Chan Khuen, Chung Yi-Li, Nayla Nasr
74.
Post-COVID-19 pulmonary fibrosis: An ongoing concern
Nuha Alrajhi
Annals of Thoracic Medicine
75.
Smoking and pre-existing co-morbidities as risk factors for developing severity of COVID-19 infection: Evidence from a field hospital in a rural area of Bangladesh
Rashadul Islam, Sayem Ahmed, Samar Chakma, Tareq Mahmud, Mamun Al, Ziaul Islam, M. Islam, Steve Zimmerman
PLOS ONE
76.
Antifibrotic Drugs against Idiopathic Pulmonary Fibrosis and Pulmonary Fibrosis Induced by COVID-19: Therapeutic Approaches and Potential Diagnostic Biomarkers
Aurelio Perez-Favila, Idalia Garza-Veloz, Lucia del Socorro Hernandez-Marquez, Edgar Fernando Gutierrez-Vela, Virginia Flores-Morales, Margarita L. Martinez-Fierro
International Journal of Molecular Sciences
77.
COVID-19 PANDEMİSİNİN YETİŞKİN BİREYLERDE SİGARA BIRAKMA BAŞARI ÖNGÖRÜSÜ ÜZERİNDEKİ ETKİSİNİN DEĞERLENDİRİLMESİ
Burcu ERGÜDEN, Yasemin ASLAN
İnönü Üniversitesi Sağlık Hizmetleri Meslek Yüksek Okulu Dergisi
78.
Risk factors associated with severe COVID-19 outcomes in Jamaica: a cross-sectional study of national surveillance data
Karen Webster-Kerr, Andriene Grant, Ardene Harris, Romae Thorpe, Daidre Rowe, Deborah Henningham, Tanielle Mullings, Iyanna Wellington, Jovan Wiggan, Kelly Ann Gordon-Johnson, Carol Lord, Tonia Dawkins-Beharie, Jemma Azille-Lewis, Jacqueline Duncan
Revista Panamericana de Salud Pública
79.
Cross-Immunity as a Potential Explanation for the Smoker's Paradox in COVID-19: Evidence from a Systematic Review and Meta-Analysis
Jesus Gonzalez-Rubio, Juan D. Navarro-López, Lydia Jiménez-Díaz, Alberto Najera
80.
Clinical Outcomes and Risk Factors for SARS-CoV-2 Breakthrough Cases Following Vaccination with BNT162b2, CoronaVac, or ChAdOx1-S: A Retrospective Cohort Study in Malaysia
Hessa Tamim, Rosnani Hashim, Nurdiana Jamil, Chong Li Yin, Zainol Johari
Heliyon
81.
Association between ABO blood groups and risk of COVID-19 infection: An umbrella review
Dorra Parv, Allahyar Shahnavazi
Journal of Hematology and Allied Sciences
82.
Cigarette smoking status and COVID-19 hospitalization in the context of cannabis use: An electronic health record cohort study in northern California
Dian Gu, Patrick Ha, Jesse T. Kaye, Michael C. Fiore, Janice Y. Tsoh
Addictive Behaviors Reports
83.
Systematic review and meta analysis of cross immunity and the smokers paradox in COVID19
Jesús Gonzalez-Rubio, Juan D. Navarro-López, Lydia Jiménez-Díaz, Alberto Najera
Scientific Reports





