INTRODUCTION
Tobacco smoking remains the leading cause of preventable deaths worldwide and poses serious public health concerns1. Although the prevalence of tobacco smoking has been steadily declining over the past decade, the number of smokers is still high2. Globally, the rate of smoking in men and women has been estimated to be 32.7% and 5.8%, respectively2. In Middle East (ME) countries, the average prevalence of tobacco use is relatively high3. The Middle East is a geographical and cultural region located primarily in south western Asia and the eastern shores of the Mediterranean Sea. The dominant cultures include Arab, Turkish, and Persian (Iranian). The region contains mainly 15 countries: Turkey, Iran, Egypt, Iraq, Lebanon, Syria, Jordan, Palestine, Kuwait, Saudi Arabia, Bahrain, Qatar, UAE, Oman, and Yemen4.
In addition to cigarette smoking, there are several other widespread types of tobacco smoking in the ME, such as waterpipe tobacco smoking (WTS) also known as hookah, shisha, arghila, and narghile, midwakh (Arabic pipe for tobacco smoking), dokha (Arabian tobacco), sniffing, and chewing tobacco. Waterpipe is the second most common tobacco product after cigarettes5. This type of smoking is very common in the ME, especially among youth and women, and this could be due to the misconception that it is less harmful than cigarettes and to the less stigma that is associated with it, which makes it an acceptable habit for women and socializing6. The prevalence of WTS among university students has been estimated to be 60.7%, 67.7%, and 63.1% in Egypt, Jordan, and Palestine, respectively7. Other countries in different parts of the region that reported lower rates of WTS among university students include the Kingdom of Saudi Arabia (KSA) (14.5%)8, Qatar (18.1%)9, and Iran (17.6%)10.
Smoking affects almost all body organs leading to a number of diseases and disabilities. It is also a risk factor for various health issues such as heart disease, cancer, stroke, chronic obstructive pulmonary disease, lung diseases, and diabetes11. Tobacco smoking is estimated to be responsible for up to 8 million deaths annually, 7 million deaths are attributed to the use of tobacco12. Tobacco smoking is not only detrimental to the smoker’s health, but also to the health of those around them since it has been documented that secondhand smoking results in significant health implications induced by inhaled smoke in the respiratory system13. Besides human health, tobacco use may also have an impact on other parameters such as national economies leading to economic destruction of hundreds of billions of dollars worldwide every year14.
According to the Centers for Disease Control and Prevention (CDC), smoking cessation services lower the rate of premature deaths and improve quality of life11. Smoking cessation can add a decade to the life expectancy of individuals who smoked previously15. The 2020 Surgeon General’s Report addressed the findings that emphasize the need to quit smoking to enhance overall health outcomes regardless of age or length of smoking16.
There are two major types of tobacco cessation (TC) interventions: non-pharmacological therapies and pharmacotherapies17. These two approaches can either be utilized separately or in combination with one another17. According to the United States Food and Drug Administration (FDA), non-pharmacological therapies include the use of behavioral treatments, such as counseling, cognitive therapy, and motivational interviewing. It can also include individual, group, and telephone counseling, all of which have been reported to be effective18. Pharmacotherapies include seven medications approved by the FDA16. These therapies have a high long-term success rate and are used to treat nicotine addiction, when patients are unable to quit or find it difficult to manage due to nicotine19. Nicotine replacement therapy (NRT) is a type of pharmacotherapy for smoking cessation. Examples include gums, nasal sprays, patches, inhalers, and lozenges. The other two nicotine-free medications are bupropion and varenicline18. Studies have shown that a combination of non-pharmacological therapies and pharmacotherapies is effective for TC16. Quitline is a population-focused tobacco cessation strategy that includes both pharmacotherapy and behavioral therapy and has been shown to have many benefits including fewer logistical obstacles and easy access such as minimizing travel costs and other costs associated with quitting smoking20.
It is imperative to note that use of theories is recommended for understanding the factors that contribute to smoking behavior and assist in developing smoking cessation interventions21. Toward this, the trans-theoretical model (TTM) is a helpful tool for assessing patients’ readiness to quit tobacco use by adopting both pharmacological and non-pharmacological therapies. TTM is composed of five main stages to motivate patients to quit: precontemplation (no plan to change within the coming 6 months), contemplation (thinking of quitting within the coming 6 months), preparation (planning to quit for the next 30 days), action (quitting successfully for less than 6 months), and maintenance (quitting successfully for 6 months or more)17.
According to the National Cancer Institute at the National Institutes of Health, smoking cessation is to quit smoking22. Smoking cessation outcomes are measured in two ways, self-report measures and biochemical validation tests. Based on consensus reached by experts, the six and/or twelve months continuous abstinence, the seven-day point-prevalence abstinence, and the number of cigarettes smoked after seven days are all important self-report measures for cessation, in addition to the biochemical test for levels of cotinine, which is a metabolite of nicotine that can be measured through urine samples23. Continuous abstinence is defined as not smoking for several months after a quitting attempt, while point-prevalence abstinence is not smoking on the day of follow-up or a couple days before.24
Several factors contribute to the success of TC programs. These are classified as multilevel factors, with individual and environmental influences predominating. Individual characteristics include smoking duration, nicotine dependence, nicotine withdrawal syndrome severity, history of failed quitting attempts, genetic factors, low self-efficacy, fear of weight gain, stress, negative mood, and depression. Social factors, tobacco marketing, and cue reactivity, are examples of environmental factors that may contribute to the effectiveness of the programs25. To help countries lower tobacco demand and supply, the World Health Organization (WHO) Framework Convention on Tobacco Control (FCTC) was established26. In all, 182 nations had ratified the FCTC as of 2020 and committed themselves to putting the suggested tobacco control measures into effect. All Middle East countries have signed the FCTC26. However, only a few of the countries have implemented or consistently enforced FCTC policies6.
Clinicians and different healthcare providers (HCPs) must be involved in TC programs, especially those who have direct contact with patients because they are in a better position to help patients quit smoking than others18. Furthermore, research has shown that it is crucial to train healthcare providers involved in TC programs to improve the outcomes of these programs27. Decision makers should also be involved, where the integration of clinicians’ efforts with healthcare systems, insurance companies, and investors, provides a chance to raise the rate of tobacco dependence treatments, quitting rates, and successful TC18.
There has been a surge in the use of validated and effective TC programs in the ME28, which has led to effective outcomes toward quitting smoking; however, no reviews have examined the specific programs used in the region. Furthermore, it is crucial to investigate the involvement of HCPs in tobacco cessation programs. This is because a study revealed a lack of specialization and training in this area in the ME29. With gaps implying a deeper and profound shortcoming in this specific area, the present systematic review aims to explore the different TC programs available in the ME and identify the factors associated with the effectiveness of these programs.
METHODS
Study design
A systematic review was undertaken using the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines (PRISMA)30. Meta-analysis was not considered in this study, since results from different studies could not be combined.
Search strategy
An electronic search of PubMed, Embase, Cochrane, ProQuest, and Web of Science bibliographic databases was conducted between 24 January 2021 and 7 March 2021, to identify all relevant studies published in peer-reviewed journals from 2000–2021. The keywords ‘Tobacco Cessation’ and ‘Middle East’ were combined using Boolean operators (AND and OR). The combination of keywords and Medical Subject Headings (MeSH) terms used in the systematic search was: [tobacco cessation OR smoking cessation OR quitting smoking OR stopping smoking OR smoking absence] AND [Middle East OR Egypt OR Palestine OR Jordan OR Lebanon OR Syria OR Qatar OR Oman OR Bahrain OR United Arab Emirates OR Saudi Arabia OR Kuwait OR Yemen OR Iran OR Turkey OR Iraq] (Table 1).
Table 1
Search combinations of keywords and MeSH terms
Eligibility criteria
Studies that met the following criteria were included:
Factors: investigating factors associated with the effectiveness of the TC program (experimental or observational);
Setting: conducted in the ME region based on the World Atlas definition for the region;
Outcome: including results of assessment of the (e.g. quitting rate); and
Language: published in the English language.
Studies with the following parameters were excluded:
Screening and data extraction
The titles and abstracts of the studies were identified and screened for relevance following the predetermined inclusion criteria (articles focusing on tobacco cessation programs and factors associated with their effectiveness), the first author uploaded the selected studies on the endnote program and removed duplicates, and if deemed eligible, only one article with comprehensive information was included in the review. During the screening process, the first two authors and the corresponding author would meet to resolve any conflict and reach an agreement. Next, a word document spreadsheet was created with the required information to be extracted. The full texts of relevant studies were accessed, and their eligibility for inclusion was evaluated.
The first two authors performed data extraction, independently. The studies were cross-checked and discussed by the two authors to resolve any disagreement. Basic information, such as author name, country, year of publication, study type, cessation methods, setting of the programs, program providers, aim of the studies, outcomes reported, and factors associated with the effectiveness of the TC programs, were retrieved from each study. All processes were performed independently; if issues arose, a discussion with the corresponding author was conducted to resolve any conflicts.
Quality assessment
The methodological quality and risk of bias of the included studies were independently assessed by two authors (MA and RA). Different tools including the Cochrane risk of bias tool for randomized controlled trials (RCTs)31, methodological item for non-randomized studies (MINORS) for quasi-experimental studies32, the National Heart Lung and Blood Institute (NIH) quality assessment tool for cross-sectional studies33, NIH quality assessment tool for pre-post-studies33, and critical appraisal skills program (CASP) for cohort studies34 specific to study design were used.
RESULTS
Study selection
The search strategy resulted in a total of 512 studies. Among these, 69 duplicates and 351 irrelevant studies were excluded. Next, of the 92 full-text assessed studies, 30 met the inclusion criteria and were included in our systematic review. A flow chart depicting the eligible studies and the reasons for exclusion of full-text articles is presented in Figure 1. The authors of some publications and conference papers were personally contacted to obtain full-text articles and information on whether the studies were peer-reviewed; some articles were delivered, and when no response was received from the authors, the study was excluded.
Study characteristics
The characteristics of the studies included in this systematic review are summarized in Table 2. We identified studies across multiple countries: Turkey (n=13), Iran (n=11), and Jordan (n=2), and one study was conducted in each of Syria, Qatar, Bahrain, and Lebanon. The included studies employed different study designs, with the majority employing a cohort study (n=12), followed by RCTs (n=9), cross-sectional studies (n=4), pre-post studies (n=3), and quasi-experimental studies (n=2). Over 50% of the studies (n=18) used both qualitative and quantitative methods for data compilation. Further, most of the included studies (79%) were conducted in TC clinics, hospitals, and universities. The sample size of the cohort studies that were published between 2007 and 2020 ranged from 100 to 34154, and 41400 were tobacco smokers. Twenty-five of the included studies reported males to be the majority of their participants, and only two studies reported almost equal representation of both genders.
Table 2
Description and characteristics of the selected studies
| Authors Country Year | Type of study | Sample size | Age of TCCs patients (years) Mean ± SD | Gender | Cessation method – behavioral change, non-pharmacological | Cessation method – pharmacotherapy | Setting | Program providers |
|---|---|---|---|---|---|---|---|---|
| Yilmaz et al.42 Turkey 2006 | Randomized controlled trial | 363 | Majority <16 | 100% females | Education and written documents about how to quit smoking | NA | Tertiary referral center | Nurses, and other personnel |
| Ybarra et al.35 Turkey 2012 | Randomized controlled trial | 150 | 36.1 ± 9.5 | 39.1% females, 60.9% males | Telephone-based counseling | NA | Local shopping malls, advertisements in local newspapers, and on Hacettepe University campus | Resident assistant |
| Heydari et al.44 Iran 2012 | Randomized controlled trial | 272 | 42.43 ± 13.4 | 41.2% females, 58.8% males | Brief phone behavior therapy counseling and education | Varenicline treatment vs nicotine replacement medications | Tobacco cessation clinics in the Tobacco Prevention and Control Research Centre | Physicians and nurses |
| Ward et al.57 Syria 2013 | Randomized controlled trial | 269 | Placebo group: 40.0 ± 11.4 Nicotine patch group: 39.9 ± 11.4 | Intervention group: 24.6% females, 75.4% males Control group: 18.5% females, 81.5% males | Face -to-face behavioral cessation counseling, and telephone support | Nicotine patches (active, placebo) | Primary care clinics | Physicians |
| Koyun and Eroğlu60 Turkey 2016 | Randomized controlled trial | 72 | Intervention group: 33.3 ± 7.7 Control: 34.0 ± 7.7 | 100% females | TTM-based training, TTM-based individual counseling, and TTMbased self-help material | NA | Family health centers | Researchers |
| Aryanpur et al.40 Iran 2016 | Randomized clinical trial | 210 newly diagnosed pulmonary TB patients with smoking habit | Majority ≥18 | Intervention group 1: 9.7% females, 90.3% males Intervention group 2: 10% females, 90% males Control group: 9.7% females, 90.2% males | Brief advice and individualized counseling session of quitting behavioral therapy | Treatment with slow- release bupropion | Six selected clinics in Tehran | Provided by one trained physician in each center |
| Orouji et al.58 Iran 2017 | Randomized controlled trial | 108 | NR | 1.8% females, 98.2% males | Educational intervention (counseling sessions) | 2 gm nicotine gum | Community-level intervention | Researchers |
| El Hajj et al.43 Qatar 2017 | Randomized controlled trial | 118 for each group | Majority >18 | 2.2% females, 97.8% males | The program is based on the trans-theoretical model of change, and tailored behavioral, cognitive and lifestyle strategies | Nicotine replacement therapy: nicotine patch | Implemented in public and private ambulatory, (17) pharmacies in the State of Qatar | Trained pharmacists |
| Durmaz et al.37 Turkey 2019 | Randomized controlled trial | 132 patients admitted to smoking cessation outpatient clinic | Majority 35–44 | 39.4% females, 60.6% males | Over the mobile phone intervention (using a messaging application) for smoking cessation and relapse prevention) | NA | Ege University Hospital, Department of Public Health, Smoking Cessation Clinic, between March and July 2017 | Healthcare provider (physicians) |
| Heydari et al.44 Iran 2010 | Quasi-experimental study | 286 | 42.4 ± 13.4 | 25.4% females, 74.6% males | ‘Cold turkey’ method and cognitive behavioral therapy | Antidepressant treatment (trazodone) and nicotine replacement | Smoking cessation clinic of Iranian National Research Institute of Tuberculosis and Lung Diseases | Physicians and nurses |
| Heydari53 Iran 2017 | Quasi-experimental study | 227 | 43.1§ | 41.6% females, 58.4% males | Information and instructions for quitting, 3 visits by the physicians in the first week, and phone call follow-up at 3 and 6 months after abstinence | Champix | Tanaffos smoking cessation clinic in Tehran | Physicians |
| Shahrokhi et al.25 Iran 2008 | Cohort study | 34154 | Majority ≥18 | Year 1998 1.8% females, 98.2% males Year 2000 2.2% females, 97.8% males Year 2002 2.6% females, 97.4% males Year 2004 9.1% females, 90.9% males | 4 Quit and Win campaigns | NA | Research center | Local sponsors |
| Heydari et al.36 Tehran 2012 | Cohort study | 308 | 42.4 ± 13.4 | 31.5% females, 68.5% males | ‘Cold turkey’ method of cessation, cognitive behavioral therapy, educational methods and consultations | 4 different types of nicotine replacement therapy (patches, chewing gum, tablets or both patches and gum) | Smoking cessation clinic of the Iranian National Research Institute of Tuberculosis and Lung Diseases | Physicians and nurses |
| Hawari et al.47 Jordan 2012 | Cohort study | 156 cancer patients | 50.3§ | 28.2% females, 71.8% males | NA | Nicotine replacement therapy with varenicline or bupropion | Quit Smoking in Cancer Center Patients in Jordan – smoking cessation clinic | Physicians |
| Hawari et al.48 Jordan 2013 | Cohort study | 201 cancer patients | 49.0§ | 19.9% females, 80.1% males | NA | Nicotine replacement therapy with varenicline or bupropion | King Hussein Cancer Center referred to the smoking cessation clinic Duration of intervention: 1 year | Medication and overall managing are provided by physicians, but counseling by a psychiatrist |
| Pekel et al.59 Turkey 2015 | Cohort study | 581 | NR | NR | Behavioral counseling | Bupropion, varenicline, NRT | Smoking cessation center | NR |
| Salepci et al.56 Turkey 2016 | Cohort study | 920 | 42.9 ± 11.5 | 40.4% females, 59.6% males | NA | Varenicline, bupropion, nicotine patches, nicotine gum | Smoking cessation clinic | Physicians |
| Turan and Turan62 Turkey 2016 | Cohort study | 179 | 35.6§ | 7.3% females, 92.7% males | Education about SC benefits, cessation process, possible withdrawal symptoms was provided | NRT, varenicline, and bupropion | Prison | Physicians and researchers |
| White et al.49 Iran 2016 | Cohort study | 100 | 40.1 ± 10.9 | 11% females, 89% males | Psychosocial support, group support | DST nicotine replacement method | Congress 60 (a recovery community in Iran) | Trained guide (achieved at least 3 months of SC) |
| Marakoğlu et al.50 Turkey 2017 | Cohort study | 3322 | 37.19 ± 12.02 | 19.2% females, 80.8% males | Smoking cessation support and behavioral therapy | Selcuk University, School of Medicine, Smoking Cessation Outpatient Clinic | Physicians, researchers | |
| Cetinkaya et al.41 Turkey 2018 | Cohort study | 857 | 18–65 | 49.8% females, 50.2% males | All participants received cognitive-behavioral therapy, 12.8% were not prescribed any medical therapy | Varenicline, bupropion, and NR | Smoking cessation clinic in Turkey | Trained physicians |
| Shoorijeh et al.38 Iran 2019 | Cohort study | 425 | 52.7 ± 16.3 | 22.8% females, 77.2% males | Phone consultation | NA | Smoking cessation program for hospital inpatients | Researchers |
| Esen et al.61 Turkey 2020 | Cohort study | 505 patient files Male: 309 Female: 196 | 38.9 ± 10.3 | 38.8% females, 61.2% males | Cognitive behavior therapy is provided by experienced specialists Counseling, and behavioral therapy during the first interview and follow-up | Using varenicline, bupropion, and NRT | Smoking cessation outpatient clinic in Turkey | Physicians |
| Heydari et al.52 Iran 2007 | Cross-sectional study | 715 | 38.3 ± 14 | 20.3% females, 79.7% males | Educational methods, consultation, cognitive-behavioral therapy | Nicotine gum | Smoking cessation clinic | Physicians |
| Hamadeh et al.54 Bahrain 2017 | Cross-sectional study | 194 | 37.2 ± 13.9 | 100% males | Counseling sessions | NRT, nicotine chewing gums and patches combined. Bupropion or champix. | Tobacco clinic only for males in Bahrain | Physicians and staff |
| Bacha et al.63 Lebanon 2018 | Crosss-ectional study | 156 enrolled patients | ≥18 years | 48.7% females, 51.3% males | 20–30 min of consultation with physiologist | NRT | Enrolled in an outpatient health center in Lebanon for three months | Respiratory physician, nurse, psychologist, dietician |
| Karadoğan et al.51 Turkey 2019 | Cross-sectional study | 417 | 44.0 ± 13.7 | 35% females, 65% males | NA | Varenicline, bupropion, and NRT | Government hospital’s smoking cessation clinic | One pulmonologist (certified for SC counseling by the Turkish Ministry of Health), one nurse, and one medical secretary |
| Sharifi et al.55 Iran 2012 | Pre-post study | 132 | Males: 37.3 ± 10.7 Females: 40.7 ± 12.2 | 12.9% females, 87.1% males | Counseling sessions | Nicotine gum | Inner-city smoking cessation clinic | Smoking counselors |
| Öztuna et al.39 Turkey 2007 | Pre-post study | 350 | 37.4 ± 11.8 | 42% females, 58% males | Counseling (face-to-face, by phone) | Pharmacotherapy (nicotine replacement therapy) | Smoking cessation clinic | Public Health and Chest Diseases departments |
Age of Tobacco Cessation Clinics (TCCs) patients is presented as mean (standard deviation). NA: not applicable. NR: not reported. NRT: nicotine replacement therapy. TTM-based: trans-theoretical model based. DST: a method of gradual treatment or reducing drug use. SC: smoking cessation. TB: tuberculosis.
The age of the participants ranged 18–69 years. The total number of participants in the RCTs was 1694, with the mean age ranging 18–80 years. These studies published between 2006–2019 were reported from Iran, Turkey, Syria, and Qatar. One RCT used a questionnaire to assess the smoking cessation rate. Most RCTs have reported the rate of nicotine dependence using the Fagerström test for nicotine dependence. In the case of the included cross-sectional studies, the sample size ranged from 417 to 1020 smokers, with 2502 participants being in the age range 13–89 years. These studies were published between 2007 and 2019. In the three pre-and-post studies that were included in the present review, the sample size ranged from 36 to 350, with age ranging 37–53 years. These were published in 2007, 2009, and 2012. The sample size of the quasiexperimental studies ranged from 227 to 286, and 513 tobacco smokers were screened. The age of the screened participants ranged 40–56 years, and the studies were published between 2009 and 2017.
Tobacco cessation programs in the ME
Eighteen studies [cohort (n=6), RCTs (n=5), cross-sectional (n=3), quasi-experimental design (n=2), before-and-after (n=2)] used both behavioral change and pharmaceutical cessation methods for TC. Four studies used only pharmaceutical cessation methods, three of which were cohort studies, and one was a cross-sectional study. Eight studies used only behavioral change cessation methods, four of which were RCTs, three included a cohort, and one was a pre-and-post study. The details are presented in Table 2.
Behavioral change cessation methods included counseling, cognitive behavioral therapy, educational materials, psychological support, physician consultation, motivational interviewing, quitting and winning, and group support. Pharmaceutical cessation methods included nicotine replacement therapy (gum and nicotine patches), varenicline, and bupropion. Five studies included quitlines as a method for behavioral counseling for tobacco cessation in Turkey and Iran, and the methods used were telephone-based counseling35, brief phone behavior therapy counseling and education36, intervention using the mobile phone (messaging application) for smoking cessation and relapse prevention37, phone consultation38, face-to-face, and phone counseling39.
Healthcare professionals providing TC programs
Various healthcare professionals provided TC programs. As seen in Table 2, the majority of studies included physicians (n=17), followed by nurses (n=6), researchers (n=6), medical secretaries (n=2), smoking counsellors (n=2), psychologists (n=1), dietitians (n=1), pharmacists (n=1), pulmonologists (n=1), and public health practitioners (n=1). Only two studies reported that physicians were trained to carry out cessation programs40,41.
Outcomes of the TC programs
The effectiveness of TC programs in most of the included studies was evaluated on the basis of the outcomes measured. The outcomes and aims of the included studies are summarized in Table 3. The most commonly reported outcome was the quit rate (n=18)36,37,40,42-56, followed by cessation rate (n=8)39,41,56-61, sustained abstinence (n=1)35, fail to quit (n=1)62, cessation survival rate (n=1)38, and success rate (n=1)35. The majority of studies utilized a self-reported questionnaire to estimate quit rates, while some others used the carbon monoxide breath test to determine quitting state (n=19)35-37,39-41,43,44,46-48,50-53, 55-57,59.
Table 3
Factors associated with tobacco cessation programs effectiveness in the Middle East
| Authors Country Year | Type of study | Aim of the study | Outcome (s) reported (%) | Factors associated with program’s effectiveness |
|---|---|---|---|---|
| Yilmaz et al.42 Turkey 2006 | Randomized control trial | To determine if mothers receiving a SC program that focuses on health risks of environmental tobacco smoking (ETS) for their kids have higher quit rate compared to mothers who received SC program that focuses on their own health, or control group mothers. | Quit rate | |
| Ybarra et al.35 Turkey 2012 | Randomized control trial | To report cessation rates observed in a messaging-base SC program for adult smokers. | Primary outcome Sustained abstinence at 3 months Secondary outcome 7- day point prevalence |
|
| Heydari et al.44 Iran 2012 | Randomized control trial | To evaluate the effectiveness of varenicline for tobacco cessation. | Quit rate 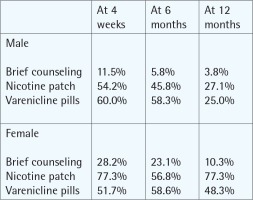 | |
| Ward et al.57 Syria 2013 | Randomized control trial | To evaluate nicotine patches and whether they boost smoking cessation rates along with behavioral support in primary health care clinics. | Cessation rates Primary end-point (prolonged)Secondary end-point (7-day point) | |
| Koyun and Eroğlu60 Turkey 2016 | Randomized control trial | To determine the influence of transtheoretical model (TTM)-based counseling, training, and a 6-month follow-up on smoking cessation in adult females. | Cessation rate At 6 months follow-up |
|
| Aryanpur et al.40 Iran 2016 | Randomized control trial | To evaluate the effectiveness of two smoking cessation methods among newly diagnosed pulmonary tuberculosis patients at the clinic. | Quit rate At 6 months |
|
| Orouji et al.58 Iran 2017 | Randomized control trial | To determine the strength of smoking cessation behavior based on a transtheoretical model. | At 6 months follow-up 40% of intervention group reached maintenance stage of smoking cessation |
|
| El Hajj et al.43 Qatar 2017 | Randomized control trial | To test the impact of a structured smoking cessation program delivered by trained ambulatory pharmacists in pharmacies. | Quit rate At 12 months | Motivation to quitSuccess to quitFailure to quit |
| Durmaz et al.37 Turkey 2019 | Randomized control trial | To evaluate the impact of support messages through WhatsApp application added to the usual care of a university hospital cessation unit, compared to usual care alone, on abstinence rates at first 4 weeks. | Quit rate At 1 month | |
| Heydari et al.44 Iran 2010 | Quasi-experimental study | To compare the effect of the four types of NRT on the quit rate. | Quite rate At 6 months At 12 months | |
| Heydari53 Iran 2017 | Quasi-experimental study | To evaluate the duration of using Champix (Varenicline) based on its cost. | Quit rate At 1 month 51.1% At 3 months 43.6% At 6 months 20.6% | |
| Shahrokhi et al.25 Iran 2008 | Quasi-experimental study | To evaluate the effect of a nursing smoking cessation intervention based on the transtheoretical model of change on a sample of military students. | Quit rate At 6 months follow-up 8.3% |
|
| Heydari et al.36 Tehran 2012 | Cohort study | To assess the efficacy of this Quit and Win contests campaign in the short-term and long-term quitting rates, and also assess some of the factors associated with quitting. | Quit rate At 1 month (self-reported) |
|
| Hawari et al.47 Jordan 2012 | Cohort study | To compare quit rates of different formulations of nicotine replacement among clients. | Quit rate After 4 weeks: 88.2% At 6 months: 54.9% At 12 months: 36.2% | After 4 weeks: 95.2% At 12 months: 62.5% |
| Hawari et al.48 Jordan 2013 | Cohort study | To measure the abstinence rates and identify reasons for the failure to quit smoking in patients visiting a smoking cessation clinic in a comprehensive cancer center. | Quit rate At 12 months was 21.2% Type of treatment and quit rate | Reasons for success
|
| Pekel et al.59 Turkey 2015 | Cohort study | To measure the abstinence rates and identify reasons for the failure to quit smoking in patients visiting a smoking cessation clinic in a comprehensive cancer center. | Quit rate At 3 months: 24.4% | |
| Salepci et al.56 Turkey 2016 | Cohort study | To establish the rate of smoking cessation and restarting in one year at the Balçova smoking cessation center. | Cessation rate At 1 year: 30.1% Relapse rate: 51.3% | |
| Turan and Turan62 Turkey 2016 | Cohort study | To compare smoking cessation rates between patients who had free medications during the period of the project, and those who had to pay for their medication. | Cessation rates At 1 month: Paid:75%, Free:43.5% (p=0.001) At 3 months: Paid:42.2%, Free:25.4% (p=0.002) At 6 months: Paid: 27.3%, Free:14.8% (p=0.008) At 1 year: Paid:18.2%, Free:12.2% (p=0.059) |
|
| White et al.49 Iran 2016 | Cohort study | To assess the smoking-related behaviors and the effectiveness of tobacco cessation therapy in prison. | Fail to quit smoking | |
| Marakoğlu et al.50 Turkey 2017 | Cohort study | To explore the introduction of a smoking cessation track within Congress 60 (a prominent recovery community within Iran). | Quit rate During the months of NRT use: 85% | |
| Cetinkaya et al.41 Turkey 2018 | Cohort study | To compare the smoking cessation rate in the 1st month, 3rd month, 6th month, 1st year, and 2nd year among those who quit smoking after taking different pharmacological and behavioral therapies. | Quit rate At 1 month: varenicline + BT users (63.5%), bupropion + BT users (49.9%), NRT + BT (53.2%), BT (17.1%) At 3 months: varenicline + BT (46.8%), bupropion + BT (35.6%), NRT + BT (24.3%), BT (7.1%) At 6 months: varenicline + BT (34.4%), bupropion + BT (28.4%), NRT (27.3.%) , BT (6.7%) At 2 years: varenicline + BT (19.9%), bupropion + BT (16.0%) |
|
| Shoorijeh et al.38 Iran 2019 | Cohort study | To know the smoking cessation rate in terms of method used to quit among patients presenting to a smoking cessation clinic in Turkey. | Cessation rate At 1 year: 34.3% |
|
| Esen et al.61 Turkey 2020 | Cohort study | To investigate the effects of smoking cessation program on inpatients and factors that may affect success. | Cessation survival rates (CSR) At 1 month: 76% At 2 months: 63% At 3 months: 61% At 4 months onward: 60% |
|
| Heydari et al.52 Iran 2007 | Cohort study | To investigate smoking cessation rates, the effects of follow-up visits and pharmacological therapies on smoking cessation in smoking cessation clinic in Turkey. | Cessation rate At 1 year: 45.3% Relapse rate: 26.8% |
|
| Hamadeh et al.54 Bahrain 2017 | Cross-sectional study | Study the correlation between nicotine dependence rate and outcome of smoking cessation among the entrants of smoking cessation clinic. | Quit rate 65.1% | |
| Bacha et al.63 Lebanon 2018 | Cross-sectional study | To determine the quit rates among male attendees of quit tobacco clinics (QTC) in Bahrain and describe related factors. | Quit rate | |
| Karadoğan et al.51 Turkey 2019 | Cross-sectional study | To assess factors associated with the success rate of smoking cessation among Lebanese smokers in a smoking cessation center. | Success rate 58.9% Failure rate 41% | |
| Sharifi et al.55 Iran 2012 | Pre-post study | To evaluate the demographic characteristics and other factors that influence the success of smoking cessation among program participants who completed a 5-year follow up. | Cessation rate After 5 years: 34.6% | |
| Öztuna et al.39 Turkey 2007 | Pre-post study | To evaluate the influence of harm reduction approach in the patterns of smoking of subjects who attended smoking cessation clinic. | Quit rate At 6 months: 12.9% |
|
[i] The table shows type of studies included in the SR, their aims, outcomes in percentage (%), and the factors associated with TC programs effectiveness. TC: tobacco cessation. HMC: Hamad Medical Corporation. NRT: nicotine replacement therapy. CO: carbon monoxide. TTM: trans-theoretical model. BT: behavioral therapy. QTC: Quit Tobacco Clinic. CSR: cessation survival rate. OR: odds ratio. CI: confidence interval
Factors associated with TC programs’ effectiveness
The various factors associated with the effectiveness of TC programs identified in this review are summarized in Table 3.
Individual factors
A number of individual factors are associated with the effectiveness of TC programs. For example, factors found to be associated with reduced effectiveness include individual withdrawal symptoms39,45,47,57, 63, high nicotine dependence rate36,38,43,49,52,60,62,63, poor knowledge on how to quit45, change in attitudes and behaviors43,56, stress47,48, number of cigarettes smoked per day62,63, inability to afford higher priced medications49,50,62, and concern about gaining weight51,63. Other individual factors that were found to be associated with effective TC programs included older age51,62, female gender61, knowledge on the benefits of tobacco cessation35,59, health issues 41,47,53,54,59,61-63, higher education level48,54, religious beliefs59, and adherence to treatment51.
Interpersonal factors
We found three interpersonal factors to be associated with TC program effectiveness: family support to quit45,54, smoking family member61, and peer support groups40. The community factors associated with the TC program’s effectiveness include motivational programs such as competition45, and psychosocial support49.
Organizational factors
The organizational factors in our review were represented by the type of services provided in TC programs. Services that were linked to effective TC programs included using NRT39-41,43,44,47,48,51, individual counseling43,54, combined programs, such as combining NRT with Cognitive Behavioral Thereby (CBT)41,46,53,58, follow-up and frequent visits to TC clinics35,37,51,54,61, long duration of treatment41, applying TTM in designing programs43,56, providing advice through health professionals45, and use of harm reduction approach in the programs55.
Environmental factors and policy
The major factors identified at the environmental and policy levels are linked to effective TC programs. These include free-of-charge treatment and cessation programs36,51,59,63, and availability of legislative tobacco control measures59. The physical environment in which the intervention was carried out plays a crucial role in influencing the effectiveness of quitting smoking; for instance, smokers in prison showed substantially lower success rates49.
Quality assessment
The results of the quality assessment are presented in Supplementary file Tables 1 to 5. According to the Cochrane risk of bias assessment tool for RCTs, only one study had a low risk of bias, while five raised some concerns of bias, and three were at a high risk of bias. Based on MINORS, for quasi-experimental studies, one comparative study scored 17 and did not reach the global ideal score for comparative studies, whereas the other two non-comparative studies scored 16 or above, which is within the global ideal score for non-comparative studies. The quality assessment tool for cross-sectional studies revealed that the majority of studies had fair to good quality since most domains constituting an integral aspect were met.
For the pre-and-post studies, the quality assessment tool showed that the blinding of outcome assessor and follow-up were not applicable; thus, the outcome may not have been accurately measured, deeming the quality of the two studies fair to poor. When the cohort studies were assessed using the CASP tool, it was noted that either most of the studies did not identify or account for confounding variables or the variables were not precise; thus, the results cannot be generalized to the local population, making the quality of these studies fair to poor.
DISCUSSION
Summary of findings
The present systematic review was performed to identify and explore the various TC programs implemented in the ME and assess the factors that influence the effectiveness of these programs. Thirty articles in ME met the inclusion criteria. The majority of the included studies employed a cohort study design, and the other 20 studies were RCTs, crosssectional, quasi-experimental, and pre-and-post. The vast majority of studies had implemented programs that integrate both pharmacological and behavioral TC interventions. While some reported that different HCPs facilitated these programs, including nurses, physicians, dietitians, medical secretaries, smoking counsellors, psychologists, pharmacists, pulmonologists, and public health practitioners, very few reported provision of training for the uptake of these programs. Meanwhile, the PHS guidelines state that the provision of more interventions and the wider the diversity of healthcare providers delivering these interventions, the more likely is that individuals will effectively quit smoking and remain abstinent64. It is imperative to emphasize the need for provider training, as this will have an additive influence on patients’ outcomes with regard to smoking cessation65.
Our systematic review identified different factors, including individual and behavioral, interpersonal, community, organizational, and environmental factors that influenced abstinence rates in TC programs. These findings provide evidence to help develop effective TC programs that encompass these factors, to improve the TC rate in the ME, as well as to support healthcare providers in planning feasible, effective, and culturally appropriate TC programs, and understand the influential factors related to the high abstinence rate in different contexts.
Factors associated with the effectiveness of TC programs
Through this review, it was clear that individual factors were associated with the effectiveness of TC programs and the success of abstinence. For example, nicotine dependence, which is regarded as an individual factor, causes various changes in the brain, making individuals feel the urge to use the substance, as a result, the appearance of a nicotine withdrawal syndrome after discontinuation increases, making quitting more difficult66. To improve the quitting success rate, healthcare professionals need to support patients by offering them behavioral treatments to control their withdrawal symptoms and overcome other obstacles. Moreover, providing insurance coverage to the population for pharmacotherapy and behavioral cessation treatments may have a significant impact on smoking cessation of individuals67. Other individual factors, such as education level, are positively associated with abstinence rates, implying that smokers with a higher level of education are more likely to quit smoking and remain abstinent47.
In addition, some studies in the ME, including Qatar and Iraq, revealed that there is a lack of knowledge regarding the dangers of tobacco smoking, necessitating the need to raise awareness about this issue68,69. The fact that a patient is concerned about being unwell or developing a health problem is a powerful individual factor associated with smoking cessation. As a result, it is worthwhile to concentrate on interventions to raise awareness about the health effects of smoking to increase the quitting rates.
A major finding of five studies in Turkey, Iran, and Bahrain, was that family pressure or support can influence the success of TC. Moreover, peer pressure ranked fourth as a reason why study participants started smoking in the first place70. On the other hand, both family members and friends have the power to positively influence and support others in quitting smoking when engaged in TC sessions71. There is evidence in the literature that successful smoking cessation is strongly associated with the absence of household members, co-workers, friends, or partners, who smoke72,73.
Motivational factors in community programs are essential to quitting smoking74. Though research on motivational factors predicts attempts to quit, it does not affect the maintenance of TC. Moreover, earlier findings have shown that motivation is not all that a smoker needs to quit74. The use of a community-wide program to provide reinforcement, support, and standards, for not smoking, is an important aspect of health-promotion activities. Additionally, individuals who live with mental health issues, such as depression or schizophrenia, are more likely to be smokers compared to healthy people, and are less likely to stop smoking or maintain a smoke-free lifestyle75. This suggests that these people require psychosocial support not only to enhance their psychological health, but also to improve health behaviors such as quitting smoking which could enhance their quality of life76. For instance, one study documented a significant reduction in the number of years in individuals with schizophrenia as a result of smoking-related diseases77. Competitions are rare in TC interventions but can be applied to support patients in the community through their journey to maintain abstinence.
Our review reported that different factors are associated with abstinence rate when it comes to services and organizational factors that were presented in TC programs. These factors can be divided into two main divisions: one being the type of treatment or therapy provided and the other healthcare providers’ training and education. Most of the programs included services such as individual counseling or CBT, NRTs, a combination of both (NRT + CBT), using harm reduction approach by professionals in the intervention, frequent follow-up in the TC clinic to stay focused on the target and help in maintenance, the application of TTM in the intervention construction, ‘cold turkey’ method, and long duration of treatment17. To enhance with evidence, we found that the combination of two different methods, such as pharmacological therapy (e.g. use of varenicline) and CBT (e.g. individual counseling), resulted in a higher score of abstinence78. For instance, according to a study conducted in Iran, the abstinence rate after 6 months in the combined intervention group was significantly higher than that in the brief advice group (71.7% vs 33.95%, p<0.001)40. Moreover, a small number of studies reported that using a quitline as a method for tobacco cessation and was only mentioned in behavioral therapies rather than pharmacotherapy or both treatments combined. In many outpatient settings, quitline services are linked to noticeably better smoking cessation efforts. Findings of an article show that integrating quitline programs within a specialist preoperative clinic may help cessation efforts79. Therefore, it is recommended to develop quitline services and interventions in the ME for better tobacco cessation outcomes79.
Physicians must play a role in improving the success rate of smoking cessation by assisting patients and providing directive instructions on management of withdrawal symptoms using several professional approaches such as counseling, interviews, discussions, and other productive methods80. In support, findings of a study done in Qatar revealed that 84.8% of respondents believed that receiving cessation advice from a healthcare expert improved the patient’s chances of stopping smoking81. In addition, trained physicians may be able to influence the views of tobacco smokers, which can increase the number of patients who seek to stop smoking and remain abstinent. It is imperative to note that trained HCPs performing tobacco cessation tasks, had a significant effect on the point prevalence of smoking, continuous abstinence, and professional performance when compared to non-trained HCPs82. Such responsibilities include creating follow-up schedules, requesting patients to set a quit date, counseling smokers’ prescriptions of a quit date, and providing self-help materials. Several health organizations throughout the world have adopted the 5As (Ask, Advise, Assess, Assist, and Arrange) smoking cessation intervention strategy, which was advocated by evidence-based smoking cessation guidelines8,9. This model is built to identify the appropriate cessation intervention based on willingness of smokers to quit smoking83. According to one study, HCPs using the 5As model had a better positive experience and felt competent in helping others to quit smoking, however, some aspects might need special training in order to be implemented for a successful tobacco cessation84.
Our review found that the presence of free-of-charge TC programs and treatments can benefit a number of individuals from various socioeconomic backgrounds, which echoes the findings from another study85. In addition to the implementation of tobacco control measures, the presence of smoke-free zones, ban on tobacco advertising, promotion, and sponsorship, sales restrictions, prohibited e-cigarette sales, and tobacco packaging and labeling, can influence the effectiveness of TC programs. According to the findings of one study, comprehensive smoke-free legislation can have a significant impact on quitting attempts86. Tobacco-free environmental policies, such as smoke-free areas and smoking bans and restrictions, will undoubtedly improve the effectiveness of TC programs and aid smoking cessation87. That is, developing an environment that supports abstinence and provides various types of assistance and encouragement for a wide range of smoking individuals, will eventually create and maintain a tobacco-free culture87.
Finally, the physical environment in which the intervention was conducted also had an impact on the program’s effectiveness. According to one study, the physical environment has a significant impact on patient abstinence rate. A study that was conducted in a Turkish prison with 38 participants, showed that 35% of those who failed to quit smoking agreed that the reason for their failed attempt was the inappropriate prison environment62. In addition, smoke-free policies are part of the physical environment factors that impact tobacco use. Private and public sector regulations that forbid smoking in indoor workspaces and in specified public areas, are both examples of smoke-free policies. Private sector smoke-free regulations may explicitly prohibit tobacco use on workplace property or limit it to specific outside areas. Community smoke-free policies set smoke-free requirements for all or specific indoor public spaces and industries88. According to Hopkins et al.88, a systematic review concluded that there is sufficient evidence to support that smoke-free policies implemented reduce tobacco use in workplaces and communities87. In addition, evidence from another systematic review shows that smoke-free policies are the best possible way to protect non-smokers from exposure to secondhand smoke89.
Strengths and limitations
To the best of our knowledge, this systematic review is the first of its kind to investigate TC programs and the factors associated with their effectiveness in ME. This review included studies with 45764 participants, which may enable generalizing the findings to the population of the ME region. Moreover, the review addresses the factors that are associated with the effectiveness of TC programs for both sexes and across different age groups. The majority of research conducted on this topic in the ME is in the form of cohort studies and RCTs, which are ranked highest in the hierarchy of evidence. Cohort studies are distinguished by their ability to measure all variables of interest and the ease with which large samples can be obtained. Likewise, RCTs are known for their ability to minimize the risk of confounding and provide the most reliable research design.
Nevertheless, the limitations of our systematic review need to be mentioned. First, the lack of data for some countries due to cultural reasons (women not smoking or underreporting of smoking status of women due to stigma) may limit the generalizability of findings to ME countries90. Second, any bias that arises as a result of publication may have an impact on our findings. Third, although our systematic review included studies from many different countries throughout the ME, not all factors could be applied equally because of heterogeneity in terms of context, environment, and culture. Heterogeneity can also be found in healthcare systems and in the availability of resources across countries, which leads to the inability to generalize the results across countries. Fourth, the included studies were of fair to poor quality according to the risk of bias assessment, emphasizing the need for conducting high-quality studies following certain guidelines based on study type for more accurate findings in the ME region. Finally, one disadvantage of RCTs is that they use self-administered questionnaires, which makes it difficult to establish causal relationships.
Implications
The findings of our review reflect gaps in the TC literature. For a comprehensive assessment of the factors affecting the effectiveness of cessation programs, future research should focus on examining the sociocultural and economic factors that might influence these programs. In addition, more research is needed to understand the barriers to seeking TC services in the ME and tackling these barriers to enhance smoking cessation in both genders. The findings also highlight the importance to conduct high-quality studies, since most of the included studies in our review were of average to poor quality according to the risk of bias assessment.
In terms of practice, our review suggests that multiple factors, including individual, interpersonal, organizational, and policy, should be considered for effective TC programs in the ME. The findings revealed that the most effective forms of cessation services were those that employed a combination of methods (pharmaceutical and behavioral change therapies). Providers of cessation programs should be trained and equipped with counseling skills to assist patients in quitting smoking, as our review revealed that trained providers had a significant positive impact on the rates of quitting smoking and abstinence. Moreover, it is equally important to examine the factors that prohibit individuals from quitting smoking while constructing or designing any TC program. Finally, establishing cost-free smoking cessation programs could help encourage more patients to quit smoking in many countries in the ME. In addition, among other services that could encourage smoking individuals to quit include visible access to smoking cessation such as using virtual counseling or mobile smoking cessation clinics, and free delivery of smoking cessation medications. Long-term follow-up studies (longitudinal and cohort research designs) with young participants are also suggested in order to assess the maintenance of tobacco cessation, by adhering to the reporting guidelines for the conducted study design so that researchers will have simple access to the crucial information.
CONCLUSIONS
The present systematic review aids in exploring different TC services and identifying the various factors that contribute to the effectiveness of TC programs implemented in the ME. Understanding these factors and how they are embedded at different levels would support healthcare providers in planning evidence-based and multilevel effective TC programs to achieve high rates of abstinence. The current findings imply the need for more focused interventions performed by trained HCPs in the ME for effective tobacco cessation. It is also advised to take into account quitline services due to their accessibility and convenience for smokers.